Electron transfer amongst flavo- and hemo-proteins: diffusible species effect the relay processes, not protein–protein binding†
Kelath Murali Manoj*a,
Sudeep K. Gadeb,
Avanthika Venkatachalamc and
Daniel A. Gideond
aSatyamjayatu: The Science & Ethics Foundation, Kulappully, Shoranur-2, Kerala, India-679122. E-mail: satyamjayatu@yahoo.com
bHemoproteins Lab, School of Biosciences and Technology, VIT University, Vellore, Tamil Nadu, India-632014
cREDOx Lab, PSG Institute of Advanced Studies, Coimbatore, Tamil Nadu, India-641004
dCIDR-MCBL, Indian Institute of Science, Bengaluru, Karnataka, India-560012
First published on 23rd February 2016
Abstract
Hitherto, electron transfer (ET) between redox proteins has been deemed to occur via donor–acceptor binding, and diffusible reactive species are considered as deleterious side-products in such systems. Herein, ET from cytochrome P450 reductase (CPR, an animal membrane flavoprotein) and horseradish peroxidase (HRP, a plant hemoprotein) to cytochrome c (Cyt c, a soluble animal hemoprotein) was probed under diverse conditions, using standard assays. ET in the CPR-Cyt c system was critically inhibited by cyanide and sub-equivalent levels of polar one-electron cyclers like copper ions, vitamin C/Trolox and superoxide dismutase. In the presence of lipids, inhibition was also afforded by amphipathic molecules vitamin E, palmitoyl-vitamin C and the membrane hemoprotein, cytochrome b5. Such non-specific inhibition (by diverse agents in both aqueous and lipid phases) indicated that electron transfer/relay was effected by small diffusible agents, whose lifetimes are shortened by the diverse radical scavengers. When CPR was retained in a dialysis membrane and Cyt c presented outside in free solution, ET was still observed. Further, HRP (taken at nM levels) catalyzed oxidation of a phenolic substrate was significantly inhibited upon the incorporation of sub-nM levels of Cyt c. The findings imply that CPR-Cyt c or HRP-Cyt c binding is not crucial for ET. Further, fundamental quantitative arguments (based on diffusion/collision) challenge the erstwhile protein–protein binding-assisted ET hypothesis. It is proven beyond reasonable doubt that mobile and diffusible electron carriers (ions and radicals) serve as “redox-relay agents” in the biological ET models/setup studied.
Introduction
Redox-active enzymes mediate electron transfer (ET) reactions amongst diverse molecules and molecular assemblies.1 Such catalytic processes are ubiquitous and they form the foundations of key metabolic and energy transduction steps, essential to all life forms. Unlike other enzymes, oxidoreductases show significant laxity in specificity and are known to be associated with the generation of “physiologically disruptive” diffusible reactive oxygen species (DROS) and diffusible radicals.2,3Variants of heme and flavin cofactor moieties are tethered to the apoprotein of redox enzymes in various modalities, which in turn affect their reactivity and selectivity.4,5 These enzymes form the mainstay of several electron transfer processes in aerobic cellular systems. Historically, the donor–acceptor protein pair of flavoenzyme cytochrome P450 reductase (CPR) and hemoprotein cytochrome c (Cyt c) have been employed to study biological ETs.6–8 The currently accepted mechanism of ET between these two enzymes vouches for a direct protein–protein complex formation, followed by long-distance electron tunneling.6–8 While investigating heme-enzyme reaction systems, we had revealed several unusual activations/inhibitions and interpreted them by invoking upon the roles of diffusible species generated within the milieu.9–17 Recently, we had alluded to the role of cyanide-based diffusible radicals to explain the inhibitions of heme-enzyme activities at low cyanide concentrations, in a regime where cyanide binding with the heme center would be a low probability event.18 We have also found that the reaction cycle of the bi-enzymatic system of liver microsomal cytochrome P450 (CYP) and CPR can be explained without entailing protein–protein complexations between the two enzymes11,13,17 (and unpublished results). Furthermore, analysis of elementary kinetics data gave us an intuitive idea that the “protein–protein binding-based ET hypothesis” appeared probable only at very high concentrations of proteins involved. These findings and arguments persuaded us to challenge the mechanism of ET in the fundamental in vitro CPR-Cyt c model. This work reveals that small diffusible species (radicals/ions) serve as relays for facilitating ETs between CPR and Cyt c under routine assay (and thereby, physiological) conditions.
Materials and methods
Peroxidase (HRP, horseradish, P6782), catalase (C9322) Cu–Zn superoxide dismutase (SOD, bovine, S2515), Cyt c (horse heart, C2506) and reduced glutathione were purchased from Sigma-Aldrich (USA). Human recombinant CPR was procured from Invitrogen (currently, ThermoFisher P2309) and cytochrome b5 (Cyt b5) was a purified laboratory preparation. Dilauroylphosphatidyl choline (DLPC) was purchased from Avanti Lipids. All other chemicals (of analytical grade, certified to be >98% purity) were procured from reputed chemical manufactures like Alfa-Aesar and Lancaster (USA), SRL and Loba-Chemie (India), etc.Flavin-based CPR quantification was done using a molar extinction coefficient of 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 at 550 nm.19 For Cyt c, the Soret band absorption was used to determine oxidized heme (ε = 106
200 at 550 nm.19 For Cyt c, the Soret band absorption was used to determine oxidized heme (ε = 106![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 at ∼410 nm) and the emergence of α band at 550 nm was used for reduced Cyt c estimation, with a molar extinction coefficient of 27
000 M−1 cm−1 at ∼410 nm) and the emergence of α band at 550 nm was used for reduced Cyt c estimation, with a molar extinction coefficient of 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600.20,21 Generally, the reactions were carried out in aerated (open) cuvettes, using potassium phosphate buffer, pH 7.4, at 27 °C. Absorption spectra and time-course data were obtained using a UV-visible spectrophotometer interfaced with a computer. To an appropriately made up reaction mixture, a suitable volume of NADPH stock was added and mixed to start the reaction. For determining the effect of ionic strength on reaction rates, the reactions contained potassium phosphate buffer at various molarities, [Cyt c] = 20 μM, [NADPH] = 20 μM, and [CPR] = 1 nM. For anaerobic reactions, the protein mixtures and NADPH solutions were flushed and maintained separately under argon for an hour, prior to NADPH addition by syringe. Specific reaction conditions are detailed in the appropriate figure legends. All data reported herein are average values (with standard deviations) of duplicates/triplicates.
600.20,21 Generally, the reactions were carried out in aerated (open) cuvettes, using potassium phosphate buffer, pH 7.4, at 27 °C. Absorption spectra and time-course data were obtained using a UV-visible spectrophotometer interfaced with a computer. To an appropriately made up reaction mixture, a suitable volume of NADPH stock was added and mixed to start the reaction. For determining the effect of ionic strength on reaction rates, the reactions contained potassium phosphate buffer at various molarities, [Cyt c] = 20 μM, [NADPH] = 20 μM, and [CPR] = 1 nM. For anaerobic reactions, the protein mixtures and NADPH solutions were flushed and maintained separately under argon for an hour, prior to NADPH addition by syringe. Specific reaction conditions are detailed in the appropriate figure legends. All data reported herein are average values (with standard deviations) of duplicates/triplicates.
Results
Elementary kinetics
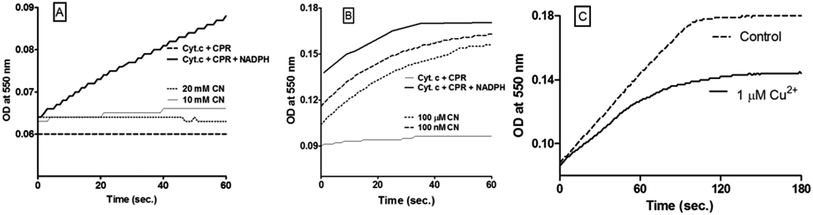
It can be noted from the positive controls in Fig. 1A and C that initial product formation rate was practically constant over a protracted time period (for very low, ∼nM level CPR concentrations and μM levels of Cyt c) and owing to this, the Cyt c reduction assay is highly reproducible. Practically, zeroth order dependence (into micromolar levels of Cyt c and NADPH) was seen in the control reactions. This is quite akin to the kinetic profiles observed in chloroperoxidase (CPO) catalyzed chlorination reactions, which is mediated via diffusible species.9 Fig. 1A shows that 10 to 20 mM level of cyanide completely causes cessation of ETs to Cyt c. At similar conditions, incorporation of azide caused only ∼46% inhibition. The relatively higher efficiency of cyanide (as inhibitor) may be explained by considering its higher redox potential. The initial absorbance values and final yields of reduced Cyt c were traced at higher amounts of reactants (CPR, Cyt c and NADPH). The results indicate that even nM to μM levels of cyanide significantly lowered productive reaction cycles (Fig. 1B). [Though the traces have similar slopes, the initial and end-point absorbance values are the points of interest here.] At higher concentrations of CPR, we would expect higher levels of radicals11 and therefore, a greater inhibitory effect by cyanide. Fig. 1B ratifies this consideration. Further, the inhibition of Cyt c reduction by cyanide was due to its impact on CPR's activity per se, and not owing to cyanide's oxidation or binding of Cyt c (Fig. S1a†). In fact, in the absence of CPR (but in the presence of NADPH), cyanide reduced Cyt c slightly, under the assay conditions (Fig. S1b†). This points out the role of cyanide-based radicals in the milieu. Also, though initial rates were not significantly altered by lowering NADPH concentration from 40 to 4 μM (in the controls), incorporation of 100 nM cyanide increased the extent of inhibition from 13% to 36% in the test reactions (not shown). This signifies that NADPH also plays an electron moderator's role in the reaction system (quite similar to peroxide's role in CPO reactions9), rather than merely serving as the electron donor to CPR. A positive reaction control in the routine assay is shown in Fig. 1C and it is compared with the time profile of the reaction with the inclusion of small amounts of copper ions. These types of rate determination with model assays (as shown in the positive controls of Fig. 1A and C; but not like those shown in Fig. 1B where CPR is in excess) were employed for the determination of pseudo-first order rates, for profiling the dose–response curves.Dose response profiles obtained upon the incorporation of various ions, molecules and enzymes
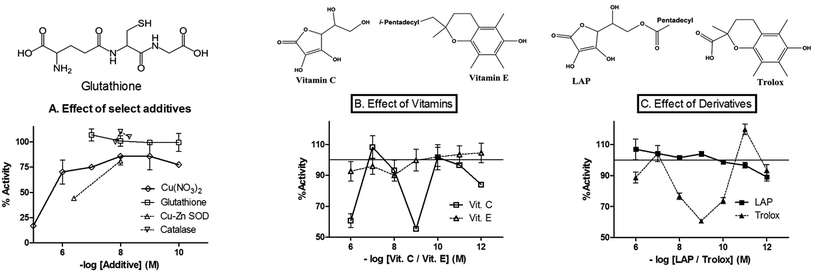
Incorporation of catalytic amounts of Cu–Zn SOD lowered the reduction rates of Cyt c (Fig. 2A). The inhibitory effect was observed even with the free solution of heat-denatured SOD. When copper sulphate and zinc sulphate were included at 100 μM concentration, the latter afforded only ∼20% inhibition; whereas, the former gave ∼95% inhibition. The inhibitory effect of Cu2+ ions (an efficient one-electron cycler) was confirmed with other salts like acetate and nitrate (data for the latter is shown in Fig. 2A). At equivalent concentrations (10 μM each Cu2+ and Cyt c), copper ions shunted away more than 80% of the electrons from CPR. This outcome cannot be explained by a CPR-Cyt c binding process. Furthermore, copper ions retained the inhibitory effect well into sub-equivalent concentrations and did not give convergence or meaningful global Ki values when plotted for non-linear regression analysis of dose–response. Fig. 2A also shows that addition of glutathione and catalase (all two-electron redox-active agents) did not significantly diminish reduction rates at sub-micromolar concentrations. (The assay with the inclusion of catalase is given in Fig. S2†). The effect(s) of incorporation (at submicromolar concentrations) of two redox-active vitamins (organic molecules) in CPR catalyzed reduction of Cyt c was studied and the results are shown in Fig. 2B. While the hydrophobic vitamin E (Vit. E) yielded very low concentration-dependent effects, vitamin C (Vit. C, a smaller and very highly water soluble molecule) showed highly concentration-dependent effects and caused profound inhibition at nM concentration ranges. To further explore this effect, derivatives of the two vitamins were incorporated (at submicromolar concentrations) in the reaction mixture and the results are given in Fig. 2C. Trolox (a water-soluble derivative of Vit. E) showed highly concentration-dependent inhibitory effects, quite similar to Vit. C. This was in stark contrast to the effect of L-ascorbic acid palmitate (LAP, an amphipathic derivative of Vit. C), which showed very little effect, quite akin to Vit. E. As noted with copper or cyanide ions, the polar redox-active organic molecules did not show the anticipated sigmoidal inhibitory dose–response profile.Effect of lipids, in conjunction with the incorporation of vitamins/derivatives or cytochrome b5, on electron transfers between CPR and cytochrome c
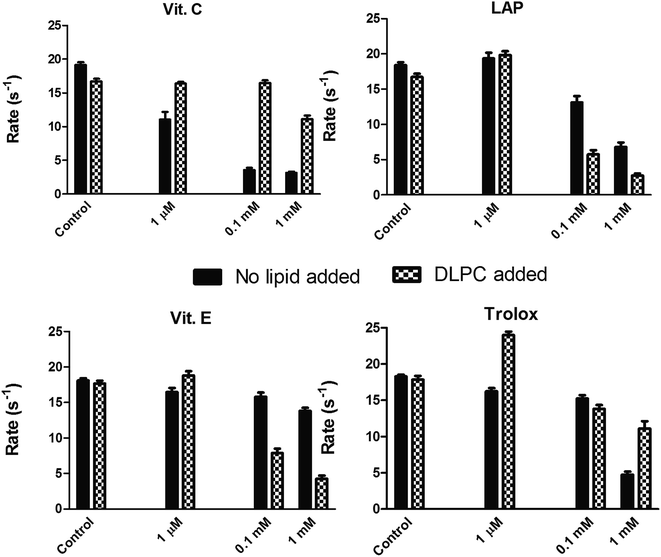
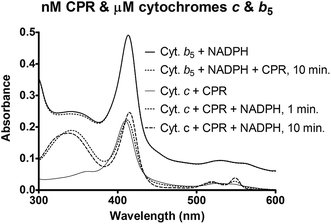
Selected vitamins and their derivatives were included at supra-micromolar concentrations in the presence of lipids, in order to probe if partitioning effects could perturb the ET reaction. The results are shown in Fig. 3. At lower concentrations of the additive, the presence of lipids alleviated inhibitions. Particularly, the hydrophobic (or amphipathic) vitamin/derivative was seen to activate the ETs marginally. At high concentrations of the additives, the presence of lipids lowered the inhibition efficiency of the water soluble vitamin C or water soluble derivative of vitamin E (Trolox). In contrast, the presence of lipids yielded higher inhibitions with excess amounts of the hydrophobic vitamin E or hydrophobic derivative of vitamin C (LAP). That is, at higher concentrations, hydrophobic additives showed more profound inhibitory effect in the presence of lipids and the water-soluble additives were more efficient (at inhibition) in the absence of lipids. This showed that ET phenomena can be rate-inhibited in both CPR-lipid microenvironment and Cyt c-aqueous microenvironment.In another experiment, ET from CPR (2–50 nM) to Cyt b5 (1–5 μM) was monitored in the presence of varying concentrations of lipid (0–100 μg ml−1). Although CPR consumed NADPH, Cyt b5 spectral signature (Soret and α-β bands) remained unchanged in these concentration regimes, within 10 minutes of mixing the reaction components (Fig. 4). This is when a much lower amount of CPR was enough to give reduction of even lower amounts of Cyt c (Fig. 4, the lower three traces). These observations showed that CPR to Cyt b5 electron-transfers/“complexations” in the lipid phase are relatively inefficient or short-lived.
In yet another experiment, the effect of inclusion of Cyt b5 (a hydrophobic cytochrome, with approximately 200 mV lower redox potential, in comparison to Cyt c) was investigated on CPR-Cyt c ET and the results are given in Table 1. (If the text that follows in this paragraph seems confusing, the reader is advised to study the data and make the interpretations directly.) Increase in lipids almost always lowers ET rates (except at 20 nM Cyt b5, for both high and low Cyt c, when lipid was raised from 10 to 40 μg ml−1). At low Cyt c, for a given Cyt b5, increasing lipid (from 0 to 400 μg ml−1) and Cyt b5 (from nil to 40 nM) lowers ET gradually and predictably. At high Cyt c, for a given Cyt b5, increasing lipid (from 0 to 400 μg ml−1) and Cyt b5 (from nil to 40 nM) lowers ET in a somewhat unpredictable but not highly significant manner. For a given lipid concentration and high Cyt c concentration, addition of Cyt b5 does not majorly perturb rates. For a lower Cyt c concentration, presence of lipids in lesser amounts lowered rates upon increasing Cyt b5 incorporation. Whereas, upon increasing Cyt b5 concentrations and in the presence of lipids in greater amounts, the rates were relatively higher. Further, for a given lipid and Cyt c concentration, there exists an optimum concentration level of Cyt b5; at low lipid – 5 nM Cyt b5 (regardless of Cyt c conc.) and at high lipid – either 5 or 20 nM Cyt b5. Presence of low or high amounts of Cyt b5 retains steady ET rates when low concentrations of Cyt c is taken, irrespective of lipid content. From 0 to 100 μg ml−1 lipid, the efficiency of increase in ET rate by ten-fold increase in Cyt c concentration remains around 2.4 ± 0.4 (when compared in analogous systems, with similar amounts of Cyt b5). At higher lipid (400 μg ml−1) and at any Cyt b5 concentrations, enhancement in ET rate was lower with ten-fold increase of Cyt c concentration.
| No. | Cyt b5↓ | DLPC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (0 μg ml−1) | (10 μg ml−1) | (40 μg ml−1) | (100 μg ml−1) | (400 μg ml−1) | |||||||
| Cyt c→ | |||||||||||
| 2 μM | 20 μM | 2 μM | 20 μM | 2 μM | 20 μM | 2 μM | 20 μM | 2 μM | 20 μM | ||
| 1 | 0 nM | 8.7 | 18.0 | 7.1 | 17.0 | 6.6 | 15.6 | 4.7 | 12.5 | 4.5 | 10.2 |
| ±0.3 | ±0.3 | ±0.3 | ±0.2 | ±0.2 | ±0.3 | ±0.2 | ±0.4 | ±0.2 | ±0.1 | ||
| 2 | 5 nM | 9.6 | 20.7 | 7.7 | 19.0 | 7.4 | 16.6 | 5.5 | 15.2 | 5.7 | 10.9 |
| ±0.2 | ±0.3 | ±0.1 | ±0.4 | ±0.2 | ±0.5 | ±0.3 | ±0.2 | ±0.2 | ±0.2 | ||
| 3 | 20 nM | 7.3 | 17.6 | 6.6 | 17.4 | 7.0 | 18.0 | 5.9 | 14.3 | 5.1 | 11.2 |
| ±0.3 | ±0.4 | ±0.1 | ±0.2 | ±0.1 | ±0.4 | ±0.1 | ±0.3 | ±0.1 | ±0.2 | ||
| 4 | 40 nM | 6.7 | 17.5 | 5.8 | 16.6 | 5.8 | 14.5 | 5.8 | 11.7 | 5.6 | 9.2 |
| ±0.1 | ±0.3 | ±0.1 | ±0.4 | ±0.2 | ±0.3 | ±0.2 | ±0.3 | ±0.3 | ±0.3 | ||
Effect of ionic strength and separation of the redox proteins by dialysis membrane
The effect of ionic strength was probed with respect to the ETs between the two proteins. Reactions were performed in distilled water (D/W) and at various ionic strengths (and the raw data are given in Fig. S3a†). The rates of Cyt c reduction in the first five minutes of incubation gave pseudo-first order (s−1) values of 10.9 ± 0.2 in a pure aqueous medium (devoid of added buffering ions) and 15.1 ± 0.6, 24 ± 0.2, 27.1 ± 0.9, 32.1 ± 0.5 and 30.8 ± 0.5 s−1, respectively, for 25 mM, 100 mM, 200 mM, 250 mM and 500 mM strengths of potassium phosphate buffer. Initial rates increase for up to 250 mM and then become slightly lower at the 500 mM range. However, when compared to higher ionic strengths (250 & 500 mM), the yield of reduced Cyt c is slightly higher at ∼15 minutes of reaction time with 100 mM ionic strength. This indicates that – (i) higher ionic strength is deleterious to enzyme stability and/or (ii) there could be competitive interactions of ions with multiple species in the milieu, which could in turn be dependent on temporally evolving concentration terms of reaction components. The important roles played by high ionic concentrations signify the role of mobile conducting species in water. A similar effect of diffusible species and excess ions was noted in the reaction milieu of CPO-catalyzed chlorinations,9 which tended to make the reaction approach zeroth order, with respect to the acceptor molecule. In order to investigate the effect of physical separation of Cyt c and CPR, the latter was confined in a dialysis tubing, thus preventing direct protein–protein complexation. From the raw data (Fig. S3b†), initial Cyt c reduction rates for various reactions were calculated, as specified in Table 2. In the D/W system, the separated reaction setup gave 14.6% activity of the positive control; whereas the buffered & separated reaction gave 5.4% activity of the respective positive control (after the values of negative control reaction rates were deducted from rates for both positive and test reactions). Under these reaction conditions, reaction in D/W slightly enhanced the yield of reduced Cyt c at longer incubation times (in positive control and in test reactions). These outcomes (very reproducible, as shown in Fig. S4†) cannot be explained by considering ionic strength as a mere requirement for optimal protein–protein complexation.| Setup | Rate (in D/W, s−1) | Rate (in buffer, s−1) |
|---|---|---|
| Positive control | 4.98 ± 0.02 | 12.83 ± 0.11 |
| Test reaction | 0.82 ± 0.03 | 0.79 ± 0.02 |
| Negative control | 0.11 ± 0.01 | 0.1 ± 0.01 |
Effect of inclusion of cytochrome c on redox reactions mediated by peroxidases-insight into electron transfer between hemoproteins
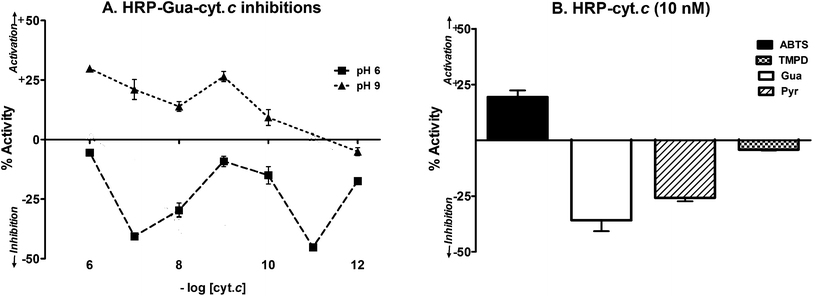
We have already shown that low concentrations of a redox-active protein like Cyt c could enhance heme-enzyme catalyzed peroxidations.16,17 (Cyt c could not affect any peroxidations on its own merit under the concentration ranges studied herein.) When HRP-Gua reactions were studied with the incorporation of Cyt c at pH 9, product formation was enhanced at most concentration ranges used. At pH 6, HRP-Gua reactions were inhibited, profoundly so at even sub-equivalent concentration ranges (Fig. 5A). When we used a stipulated concentration of Cyt c (10 nM) while keeping peroxide and HRP at identical concentrations (Fig. 5B), it was seen that the effect was dependent on the nature of the substrate. While ABTS peroxidation was significantly enhanced by Cyt c and TMPD peroxidation was marginally inhibited, the product formation from phenolics Gua and Pyr were significantly inhibited by >25% of control.Discussion
Heme iron in Cyt c is hexacoordinated. Though the axial methionyl ligation in Cyt c could be displaced to give a cyanide-coordinated protein species, it occurs significantly only at very high concentrations (0.1 M) of cyanide.22 The inhibitions by low concentrations of diverse species such as metal ions, cyanide/azide anions, enzymes and organic molecules etc., reported in the current manuscript, cannot be attributed to such displacement of coordination spheres. CPR is not known to “bind” cyanide and is known to transfer electrons to a wide variety of enzymes (heme oxygenase, fatty acyl elongase, squalene monooxygenase, cholesterol reductase etc.), several cytochromes (hundreds of P450 isozymes, b5, c etc.) and a bevy of natural organics and synthetic dyes. It is difficult to envisage that CPR has topological and electrostatic binding complementarities with all of these redox active molecules (particularly negatively charged ions and small organic molecules). If that were the case, we should not have found critical dependence on ionic strength and maverick modulations by enzymes/small organic molecules/metal or inorganic ions. The present work does not challenge the existence or solved crystal structures of complexes between various redox proteins (like cytochrome P450 and CPR). However, it is doubtful that these complexes hold any significance in the physiological or in vivo/in vitro assay scenarios. Till date, we find no direct/conclusive evidence for donor (CPR)–acceptor (Cyt c) complexes' contributory role in ETs in dilute aqueous solutions or low mobility scenarios (like – within phospholipid membranes).The diverse diffusible ionic or molecular or radical species (and enzymes like SOD) can inhibit or enhance the electron shuttling mechanism between CPR and Cyt c. This finding is along the lines that we had demonstrated in our recent work on heme peroxidase catalysis.18 Therefore, the promiscuity of CPR and non-conformity to classical dose–response profiles is explained by the intermediary role of diffusible species. Similar effects are afforded by molecules that have comparable molecular parameters (as exemplified by the parameters for solubility and partitioning, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P and log
P and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D, Fig. 2B and C). Partitioning effects are clearly evident in the data shown in Table 1 and Fig. 2 and 3. It can be seen from Fig. 3 that in the absence of lipid, an excess of the polar molecule (≥10 μM of vitamin C being a salient example; Trolox is relatively less polar and therefore, requires a greater excess, ∼1 mM) is more efficient in inhibiting CPR-Cyt c reaction. In contrast, when lipid is added, excess of the hydrophobic/amphipathic molecules (LAP and vitamin E) are more effective in inhibiting the CPR-Cyt c ET process. This is because the addition of lipid partitions CPR solely into the lipid phase and therein, the excess of redox active hydrophobic molecules have greater sway to access and dissipate the electrons. Secondary catalytic activity mediated by additive-based radicals alone can explain why – (a) 100 nM levels of Vit. C or Trolox do not inhibit ET, but 1 nM levels of these molecules inhibit ET significantly (Fig. 2B and C) & (b) sub-equivalent levels (1–100 pM) of Vit. C or Trolox significantly perturb (activate or inhibit) the catalysis by nM concentrations of CPR (Fig. 2B and C). Increasing the concentration of a radical scavenger not only promotes its competition with Cyt c; additionally, the scavenger's catalytic ability to dissipate the electrons unproductively could also be affected (because they collide more frequently amongst themselves, and collapse). Also, the redox active molecule/ion/radical species may (catalytically) reduce Cyt c on its own merit, which could explain some of the activations observed.14–17 Since small amounts of water-soluble and highly mobile radical cyclers (but not hydrophobic ROS scavengers) critically affect ETs between CPR-Cyt c, the rate limiting/essential process in these regimes (in the absence of lipids) is understood to be ‘diffusion-based’ in water. These inferences are corroborated and complemented/supplemented from our own studies involving the CPR-CYP catalytic system (both hydrophobic proteins, co-localized in the phospholipid membrane interface).17 In these systems (subjected to diffusion in the phospholipid microenvironment), the reactions were drastically inhibited by sub-micromolar concentrations of the amphipathic/hydrophobic and bulky DROS scavengers, and not the soluble vitamins or derivatives.17
D, Fig. 2B and C). Partitioning effects are clearly evident in the data shown in Table 1 and Fig. 2 and 3. It can be seen from Fig. 3 that in the absence of lipid, an excess of the polar molecule (≥10 μM of vitamin C being a salient example; Trolox is relatively less polar and therefore, requires a greater excess, ∼1 mM) is more efficient in inhibiting CPR-Cyt c reaction. In contrast, when lipid is added, excess of the hydrophobic/amphipathic molecules (LAP and vitamin E) are more effective in inhibiting the CPR-Cyt c ET process. This is because the addition of lipid partitions CPR solely into the lipid phase and therein, the excess of redox active hydrophobic molecules have greater sway to access and dissipate the electrons. Secondary catalytic activity mediated by additive-based radicals alone can explain why – (a) 100 nM levels of Vit. C or Trolox do not inhibit ET, but 1 nM levels of these molecules inhibit ET significantly (Fig. 2B and C) & (b) sub-equivalent levels (1–100 pM) of Vit. C or Trolox significantly perturb (activate or inhibit) the catalysis by nM concentrations of CPR (Fig. 2B and C). Increasing the concentration of a radical scavenger not only promotes its competition with Cyt c; additionally, the scavenger's catalytic ability to dissipate the electrons unproductively could also be affected (because they collide more frequently amongst themselves, and collapse). Also, the redox active molecule/ion/radical species may (catalytically) reduce Cyt c on its own merit, which could explain some of the activations observed.14–17 Since small amounts of water-soluble and highly mobile radical cyclers (but not hydrophobic ROS scavengers) critically affect ETs between CPR-Cyt c, the rate limiting/essential process in these regimes (in the absence of lipids) is understood to be ‘diffusion-based’ in water. These inferences are corroborated and complemented/supplemented from our own studies involving the CPR-CYP catalytic system (both hydrophobic proteins, co-localized in the phospholipid membrane interface).17 In these systems (subjected to diffusion in the phospholipid microenvironment), the reactions were drastically inhibited by sub-micromolar concentrations of the amphipathic/hydrophobic and bulky DROS scavengers, and not the soluble vitamins or derivatives.17
Table 1 shows that the radicals generated by CPR (in the lipid phase) need to reach Cyt c (in the aqueous milieu) and for the same, there is an optimal lipid/Cyt b5 paradigm. If excess lipid or Cyt b5 was present, the probability increases that the electrons are sequestered or dissipated to water formation. If the amount is optimal, the ET is optimally phased and smooth. The concept that “protein–protein collisions lead to binding or result in conformation changes of redox proteins and therefore, enhancement of catalytic activity”23,24 makes little justice to scientific logic or experimental data. If the erstwhile paradigm were true, then an increase in Cyt b5 should only give an increase in ET in all CPR-Cyt c ETs or higher concentrations of Cyt b5 should only enhance all CYP reactions. But this is not seen. In contrast, in spite of possessing a favorable redox potential (with respect to CPR) and being co-localized in the lipid environment, Cyt b5 does not get noticeably reduced (Fig. 4). This shows that protein–protein collisions are a relatively slow phenomenon in lipid membranes (and/or that Cyt b5 cannot be the stable/terminal electron acceptor in the system), with respect to the mechanistic phenomena involved in the kinetics of ET.
The observation that ETs occurred even after physical separation of the two proteins confirms the inference that protein-binding is not obligatory for electron transfer. It is demonstrated from our works that CPR initiates a radical reaction,11 which is subsequently relayed to Cyt c. Oxygen is not obligatory for this step (as we have noted that electron transfers to Cyt c occurs even in anoxic conditions) and ionic conductivity facilitates this relay step. Redox-active additives can enhance/lower this process. The concentration of reduced Cyt c goes up in the milieu, only because this species is more stable (owing to the dissipation of electron into the heme/apoprotein and owing to the high redox potential) than the other “transiently reducible” components within the reaction system. As of now, it is not facile to characterize the exact nature of diffusible species involved, for the following simple reasons – (i) the diffusible species are short-lived, (ii) their effective concentrations would be in sub-μM to supra-pM ranges and (iii) these species can be envisaged to be in dynamic equilibrium with diverse species in aqueous milieu, and each pathway could lead to chaotic outcomes. Till date, there are no physical or chemical probes available to pinpoint the dynamics in such systems accurately/precisely.
The argument that – “The physically separated reaction setup could only give ∼10–15% of control's activity and therefore, the primary route is via protein–protein complexation” is misplaced. This is because radicals like superoxide have high auto-collapse (dismutation) rates, with second order rate constants of 105 M−1 s−1.25 If an ‘electron-relaying species’ does not encounter a molecule of Cyt c in its immediate vicinity, the electron is taken away by other interactive components in the milieu, leading to two electron processes and “non-productive” water formation. Such a scenario is also involved in the CPO reaction milieu, where, the reactive intermediate is consumed by peroxide itself, if a suitable substrate is not present.9 Therefore, separation by the membrane drastically decreases Cyt c reduction rates in comparison to the freely mixed system.
Calculations and experimentations show that the diffusion rate of a small molecule like oxygen in water (diffusion coefficient, D = 2000 μm2 s−1) is ∼102 to 103 times faster than the diffusion rates of a protein in cytoplasm (D = 20 to 2 μm2 s−1) and ∼104 to 105 times the rate of diffusion of a protein on phospholipid membranes (for which, D is approximately 0.2 to 0.02 μm2 s−1).26 Optimized ET rates between CPR and Cyt c afforded pseudo-first order rates of 32 s−1 or above. This would mean an overall second order rate constant of ∼107 M−1 s−1 (when considering dependence on Cyt c into micromolar ranges) or >1010 M−1 s−1 (with dependence on nM levels of CPR as the rate determining component, in the reaction assays). The latter value is apparently erroneous because the second order diffusion limitations of small molecules in water is ∼109 M−1 s−1. If we multiply it by the concentration of CPR, we get the collision frequency – which is 1 per second, about an order short of the actually observed rates. When considering that we are dealing with two dilute and bulky proteins that are housed/distributed micro-heterogeneously (nM levels of CPR housed in lipids with μM levels of Cyt c in aqueous phase), we can never envisage such super-efficient collisions of bulky proteins. If we factor in the lower mobility of bulky proteins stationed in the plasma membranes and/or cytoplasm, the rates afforded by protein–protein complexation mediated ET would fall short of the observed rates by five to seven orders of magnitude! On the contrary, if we consider that the reaction occurs between diffusible radical species and Cyt c, then the higher concentration of the much faster species at an instant would result in a lower second order rate calculation or more facile collision frequencies. We can now see that it is improbable that collisions between proteins occurring in lipid phase (a low energy environment) could ever account for the electron transfer phenomenon under study. There is little reason to argue that CPR would give electrons only to another protein.11 Also, only the presence and obligatory involvement of significant amounts of highly active diffusible species alone can explain how low amounts of redox-active proteins (like cytochromes c and b5) can significantly modulate ET rates (between proteins) and impact oxidation efficiency of several small molecules (in reactions mediated by heme proteins). At the acidic pH ranges we studied, HRP-Gua reactions were inhibited with sub-nM levels of Cyt c (Fig. 5A), when HRP was taken at 1 nM concentration. Two bulky proteins at nanomolar levels cannot collide with enough frequency to bring about an alteration of electron transfer rates in the timescales carried out in our experiment. Cyt c is an animal protein and HRP is a plant protein and there is no rhyme or reason for any affinity binding between the two either. Even if there was a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 tight binding under these conditions (assuming the most improbable scenario!), it should only knock out 1–2% of activity, and cannot give such high inhibition. Therefore, the shunting of electrons between HRP-Cyt c must occur via diffusible species. As a logical consequence, the one-electron transfers must occur via the route – CPR to diffusible species to Cyt c. This deduction is confirmed by the fact that Cyt c inhibited HRP mediated electron abstractions from guaiacol (Fig. 5A) in a rather maverick concentration-dependent fashion, quite akin to Vit. C or Trolox inhibiting ET in the CPR-Cyt c couple (Fig. 2B and C). This showed that the ET phenomenology in such systems is generic and not specific. This electron relay is quite similar to the well-established processes in aqueous electrochemistry wherein ions relay the charges under an applied potential or when two half-cells of varying redox potentials are connected via a salt-bridge. In the “biological” relay studied herein, there is a lot of room for uncoupling too (to form water), which quenches the “transfer” process. This is why the receiving element must be located close by to the donating element, which is effectively achieved by having them co-immobilized on phospholipid membranes or by solubilizing them homogeneously in the aqueous phase.
2 tight binding under these conditions (assuming the most improbable scenario!), it should only knock out 1–2% of activity, and cannot give such high inhibition. Therefore, the shunting of electrons between HRP-Cyt c must occur via diffusible species. As a logical consequence, the one-electron transfers must occur via the route – CPR to diffusible species to Cyt c. This deduction is confirmed by the fact that Cyt c inhibited HRP mediated electron abstractions from guaiacol (Fig. 5A) in a rather maverick concentration-dependent fashion, quite akin to Vit. C or Trolox inhibiting ET in the CPR-Cyt c couple (Fig. 2B and C). This showed that the ET phenomenology in such systems is generic and not specific. This electron relay is quite similar to the well-established processes in aqueous electrochemistry wherein ions relay the charges under an applied potential or when two half-cells of varying redox potentials are connected via a salt-bridge. In the “biological” relay studied herein, there is a lot of room for uncoupling too (to form water), which quenches the “transfer” process. This is why the receiving element must be located close by to the donating element, which is effectively achieved by having them co-immobilized on phospholipid membranes or by solubilizing them homogeneously in the aqueous phase.
The aesthetic argument – “Life could not have evolved with biological ET based on diffusible species (via a radical or one-electron process), because such a process would be chaotic” does not hold merit. Life emerged from chaos and therefore, its fundamental signature is written at the basis of life sustaining process of energy transduction (through ET). In mitochondrial membranes, ubiquinone (a soluble small molecule) is known to play indispensable role as a two-electron shuttling agent. Therefore, it is not obligatory that one-electron transfer in cells is exclusively mediated via a protein–protein transaction. It has already been demonstrated that enzymatic redox reactions mediated by diffusible species could be highly selective and reproducible.9 We have shown that both theoretical considerations and experimental findings clearly point out that the erstwhile aesthetic considerations are misplaced here. Though the diffusible species mediate electron transfer, they do it in an efficient and highly reproducible manner (the impeccably small standard deviations in the rate calculations are a testimony to the same). It is proven beyond reasonable doubt that ET phenomena in CPR-Cyt c (Cyt b5) systems is mediated through a relay of small molecules/ions/radicals. The projections we had made earlier that DROS serves as the electron transfer agent between diverse CYPs and CPR is hereby ratified.11–13,17 Therefore, this work lends solid support to the ‘murburn’ hypothesis27 proposed to explain CYP + CPR mediated metabolism of xenobiotics in liver microsomes. Also, we can now understand how Cyt b5 can serve as a “transient buffer” of one-electron equivalents in a CYP-CPR reaction system. The findings reported herein serve to explain the promiscuity of redox enzymes like CPR and further explicate the physiological toxicity of low amounts of agents like cyanide (species that are lipid soluble and have high redox potentials/mobility18). The findings usher in a new paradigm in cellular redox biochemistry. Now, the diffusible radicals and ROS species cannot be seen merely as redox signaling molecules or manifestation of patho-physiological states alone. They are now understood as an obligatory requirement of routine oxidative metabolism in such membrane systems involving heme/flavin proteins.
Acknowledgements
KMM dedicates this manuscript to late Lowell P. Hager (UIUC, Member – NAS, USA). KMM thanks Abhinav Parashar (VIT University) for assistance in preparation of the manuscript. The work was powered by Satyamjayatu: The Science & Ethics Foundation.References
- I. Fridovich, L. B. Poole, A. Holmgren, M. F. Lou, V. N. Gladyshev, S. S. David, R. L. Osborne, J. H. Dawson, S. D. Copley, H. Kadokura, J. Beckwith, H. F. Gilbert and S. W. Ragsdale, in Redox Biochemistry, John Wiley & Sons, Inc., 2007, pp. 49–134 Search PubMed.
- R. C. Zangar, D. R. Davydov and S. Verma, Toxicol. Appl. Pharmacol., 2004, 199, 316–331 CrossRef CAS PubMed.
- A. Naqui, B. Chance and E. Cadenas, Annu. Rev. Biochem., 1986, 55, 137–166 CrossRef CAS PubMed.
- T. L. Poulos, Chem. Rev., 2014, 114, 3919–3962 CrossRef CAS PubMed.
- D. Becker, C. Binda, E. Ceccarelli, P. Chaiyen, A. J. C. Filho, B. Daniel, C. Dully, D. Edmondson, P. Fitzpatrick and G. Gadda, Handbook of Flavoproteins Volume 1 Oxidases, Dehydrogenases and Related Systems, De Gruyter, Berlin, 2013 Search PubMed.
- M. Wang, D. L. Roberts, R. Paschke, T. M. Shea, B. S. S. Masters and J.-J. P. Kim, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 8411–8416 CrossRef CAS.
- C. Kenaan, H. Zhang, E. V. Shea and P. F. Hollenberg, Biochemistry, 2011, 50, 3957–3967 CrossRef CAS PubMed.
- M. Sugishima, H. Sato, Y. Higashimoto, J. Harada, K. Wada, K. Fukuyama and M. Noguchi, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 2524–2529 CrossRef CAS PubMed.
- K. M. Manoj, Biochim. Biophys. Acta, 2006, 1764, 1325–1339 CrossRef CAS PubMed.
- K. M. Manoj and L. P. Hager, Biochemistry, 2008, 47, 2997–3003 CrossRef CAS PubMed.
- K. M. Manoj, S. K. Gade and L. Mathew, PLoS One, 2010, 5, e13272 Search PubMed.
- K. M. Manoj, A. Baburaj, B. Ephraim, F. Pappachan, P. P. Maviliparambathu, U. K. Vijayan, S. V. Narayanan, K. Periasamy, E. A. George and L. T. Mathew, PLoS One, 2010, 5, e10601 Search PubMed.
- D. A. Gideon, R. Kumari, A. M. Lynn and K. M. Manoj, Cell Biochem. Biophys., 2012, 63, 35–45 CrossRef CAS PubMed.
- D. Andrew, L. Hager and K. M. Manoj, Biochem. Biophys. Res. Commun., 2011, 415, 646–649 CrossRef CAS PubMed.
- A. Parashar and K. M. Manoj, Biochem. Biophys. Res. Commun., 2012, 417, 1041–1045 CrossRef CAS PubMed.
- S. K. Gade, S. Bhattacharya and K. M. Manoj, Biochem. Biophys. Res. Commun., 2012, 419, 211–214 CrossRef CAS PubMed.
- A. Parashar, S. K. Gade, M. Potnuru, N. Madhavan and K. M. Manoj, PLoS One, 2014, 9, e89967 Search PubMed.
- A. Parashar, A. Venkatachalam, D. A. Gideon and K. M. Manoj, Biochem. Biophys. Res. Commun., 2014, 455, 190–193 CrossRef CAS PubMed.
- J. L. Vermilion and M. J. Coon, J. Biol. Chem., 1978, 253, 2694–2704 CAS.
- V. P. Miller, G. DePillis, J. Ferrer, A. G. Mauk and P. O. De Montellano, J. Biol. Chem., 1992, 267, 8936–8942 CAS.
- E. Margoliash and N. Frohwirt, Biochem. J., 1959, 71, 570 CrossRef CAS PubMed.
- A. Schejter, M. D. Ryan, E. R. Blizzard, C. Zhang, E. Margoliash and B. A. Feinberg, Protein Sci., 2006, 15, 234–241 CrossRef CAS PubMed.
- C. W. Locuson, L. C. Wienkers, J. P. Jones and T. S. Tracy, Drug Metab. Dispos., 2007, 35, 1174–1181 CrossRef CAS PubMed.
- S. Kumar, D. R. Davydov and J. R. Halpert, Drug Metab. Dispos., 2005, 33, 1131–1136 CrossRef CAS PubMed.
- S. Marklund, J. Biol. Chem., 1976, 251, 7504–7507 CAS.
- R. Milo and R. Phillips, Cell Biology by the Numbers, Garland Science, Taylor & Francis Group, LLC, New York, 2015 Search PubMed.
- A. Venkatachalam, A. Parashar and K. M. Manoj, In Silico Pharmacol., 2016, 4(1), 1–38, DOI:10.1186/s40203-016-0016-7.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra26122h |
| This journal is © The Royal Society of Chemistry 2016 |