Eco-benign visible wavelength photodetector based on phthalocyanine-low bandgap copolymer composite blend
Abstract
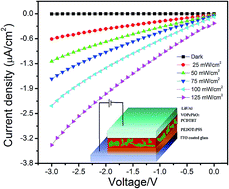
Herein, we demonstrate a novel solution-processed photodetector using an organic composite blend of VOPcPhO and PCDTBT to function as the donor (D) and the acceptor (A) materials, respectively. The absorption spectra of the composite blend have been successfully broadened to encompass almost the entire visible region by exploiting the spectral properties of PCDTBT moiety. The photodetector shows an efficient photo-current response as a function of varied illumination levels. Furthermore, current density–voltage (J–V) characteristics of the device, in the dark, have been employed to extract device parameters such as barrier height and rectification ratio. The conduction mechanisms at the electrode–semiconductor interface have also been investigated. As the proposed photodetector can harvest incident photons over almost the whole visible range, it can be potentially employed for practical applications.

 Please wait while we load your content...
Please wait while we load your content...