Controlled synthesis of pyrochlore Pr2Sn2O7 nanospheres with enhanced gas sensing performance†
Qi Liu*a,
Miao Xua,
Ze-Xian Lowc,
Wen Zhanga,
Feng Taoa,
Feng Liub and
Ning Liua
aDepartment of Materials Science and Engineering, Anhui Polytechnic University, Wuhu, Anhui 241000, P. R. China. E-mail: modieer_67@ahpu.edu.cn; Fax: +86553 2871 252; Tel: +86 553 2871 252
bCollege of Mathematics and Physics, Anhui Polytechnic University, Wuhu 241000, P. R. China
cDepartment of Chemical Engineering, University of Bath, Claverton Down, Bath BA2 7AY, UK
First published on 9th February 2016
Abstract
Uniform Pr2Sn2O7 nanospheres were synthesized via a simple solvothermal route with ethylenediamine as the solvent. The as-prepared Pr2Sn2O7 nanospheres with the diameters of 20–50 nm are composed of nanoparticles with a size of 3–5 nm. The introduction of water into the reaction solution results in the formation of smaller Pr2Sn2O7 nanospheres with poor crystallinity or SnO2 particles, indicating that ethylenediamine has a complicated effect on the generation of Pr2Sn2O7 nanospheres. The UV-vis spectrum reveals that the band gap of synthesized Pr2Sn2O7 nanospheres is about 4.19 eV, much higher than the theoretical value calculated by DFT. The enhanced gas sensing performances could be attributed to the unique mesoporous nanosphere structure, which can significantly facilitate gas diffusion and mass transportation in sensing materials.
Introduction
The physical and chemical performance of materials are determined not only by their chemical composition, but also by their physical dimensions, structures, shapes and sizes.1–3 In recent decades, considerable progress has been made in size- and shape-controlled synthesis of nanocrystals. Nanoporous spheres have attracted a great deal of attention because of their unique structural, physical and chemical properties such as high specific surface area, low density, and good permeation.4–7 Hence, the fabrication of nanospheres with pore sizes ranging from one to hundreds of nanometers is critically important for both scientific research and technological development.Due to their catalytic activity, defect structures, unique mechanical properties and high thermal stability,8–11 lanthanide stannate pyrochlores Ln2Sn2O7 (Ln = lanthanide elements) with cubic pyrochlore structure (Fd3m) have emerged as prospective materials for different applications such as catalysts, dielectrics, fast ion conductors, superconductors, ferromagnets and hosts for radioactive waste.8–11 The conventional method for the preparation of Ln2Sn2O7 is a solid-state reaction by calcining tin oxide with lanthanide oxide at high temperature (about 1500 °C) for a long time (up to 5 d).12–16 The obtained powders usually show extensive aggregation and compositional inhomogeneity, which degrades their performance. Recently, much emphasis has been paid on developing low-temperature liquid phase methods to get nanosize pyrochlore structures with shape and size control.17–19 However, Sn4+ ions are easily hydrolyzed and there are distinct differences between Sn4+ and Ln3+ of physicochemical properties. It is difficult to prepare Sn-based pyrochlores via wet chemical approaches. Only few Ln2Sn2O7 nanocrystals have been successfully synthesized via facile hydrothermal methods with good performance such as high photocatalytic activity,18,19 ionically conducting properties at high temperatures.17
Among the lanthanide stannates, Pr2Sn2O7 was reported as a new dynamic spin ice.15,16 Due to the large ionic radius and small moment of Pr3+, pyrochlore oxide Pr2Sn2O7 is the most promising candidates for observation of strong quantum effects.13 The conventional preparation method of Pr2Sn2O7 is a solid-state reaction.13,15,16 To the best of our knowledge, there is no report on the preparation of Pr2Sn2O7 pyrochlore nanocrystals by wet chemical approaches and their application in gas-sensing. In this study, stoichiometric Pr2Sn2O7 nanospheres were prepared by a facile solvothermal method with ethylenediamine (en) as the solvent. The synthesized Pr2Sn2O7 nanospheres (ca. 20–50 nm) consist of nanoparticles with a size of 3–5 nm. To demonstrate the potential applications, the gas-sensing properties of Pr2Sn2O7 nanospheres were investigated. A comparative gas sensing study between the as-synthesized Pr2Sn2O7 nanospheres and SSR-Pr2Sn2O7 was performed to demonstrate the enhanced gas sensing properties of the 3D porous Pr2Sn2O7 materials.
Experimental
Preparation of the Pr2Sn2O7 particles
All the chemicals were of analytical grade and used as received without further purification. The Pr2Sn2O7 nanospheres were synthesized by a simple solvothermal route with en as the solvent. In a typical synthesis, 0.318 g of Pr(CH3COO)3 (1 mmol) and 0.351 g of SnCl4·5H2O (1 mmol) were added to 15 mL en. The mixture was stirred for 40 min and then transferred to a stainless steel, Teflon-lined autoclave of 25 mL inner volume. Solvothermal synthesis was performed under an auto-generated pressure at 180 °C for 24 h in an electric oven, followed by natural cooling to room temperature. The product was collected by centrifugation, washed thoroughly with deionized water and ethanol for 3 times, respectively, and then dried at 80 °C for 12 h.Characterization
The crystallographic phase of these as-prepared products was determined by an X-ray diffractometer (XRD) (Rigaku Ultima III, Japan) using Cu Kα radiation (λ = 0.154178 nm) at 40 kV and 40 mA. The XRD patterns were obtained over the scanning range of 10–80° at room temperature with a scan rate of 10° min−1. The morphology of the powders was examined by field emission scanning electron microscopy (FESEM, FEI NOVA NANOSEM 230). Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) images were obtained on a JEOL JEM-2100 microscope with a LaB6 filament and an accelerating voltage of 200 kV. The UV-vis diffuse reflectance spectrum was recorded with a UV-vis spectrophotometer (UV-2550, Shimadzu) at room temperature and transformed to the absorption spectrum according to Kubelka–Munk relationship. The specific surface area of the samples was measured by nitrogen sorption at 77 K on a surface area and porosity analyzer (Micromeritics TriStar 3000, USA) and calculated by the BET method.Gas-sensing test
Pr2Sn2O7 samples were mixed with deionized water to form pastes. The pastes were coated onto the surface of an Al2O3 microtube with four Pt electrodes. The coating process was repeated several times to obtain the desired coating. Then the coated Al2O3 microtube was welded on a pedestal with six poles. And a heating coil was inserted through the Al2O3 microtube and its two ends were welded to the other two poles of the pedestal. The ethanol sensing characteristics of the prepared sensors were measured using a JF02E gas sensor test system (Kunming Guiyan Jinfeng technology Co. Ltd). Different concentrations of ethanol vapors (100–600 ppm) were used as the target gas to test the sensing performance of the samples. The time taken by the sensor to achieve 90% of the total resistance change was defined as the response time in the case of adsorption and the recovery time in the case of desorption.Results and discussion
Typical XRD pattern of the as-prepared sample with reaction time of 24 h is shown in Fig. 1a. All of the X-ray diffraction peaks are indexed to the cubic pyrochlore-type Pr2Sn2O7 (JCPDS13-0184) with the lattice constant a = 1.0604 nm, which is in good agreement with the standard card. No impurity peaks such as those of SnO2 or Pr(OH)3 were detected, indicating the phase-pure Pr2Sn2O7 of the product. This result indicates that the crystalline Pr2Sn2O7 can be formed using the aforementioned facile solvothermal method at a relatively low temperature. The crystal structure of Pr2Sn2O7 material is also shown in Fig. 1b and c. The crystalline has the cubic pyrochlore structure with space group Fd3m and contain eight formula units per unit cell. The Pr ions (III) are surrounded by eight oxygen atoms in a distorted cubic polyhedron, with the Sn IV ions coordinated by six oxygen atoms in an octahedron configuration. | ||
| Fig. 1 (a) XRD pattern of the Pr2Sn2O7 crystalline, and cubic pyrochlore crystal structure of the Pr2Sn2O7 crystalline: (b) the ball-and-stick model, (c) the polyhedron model. | ||
Morphologies of the samples were examined by FE-SEM at room temperature, as shown in Fig. 2. The lower magnification of the FE-SEM images in Fig. 2a clearly show that a typical sample has a hierarchical structure of colloidal spheres with a diameters between 20–50 nm. These spheres were formed from numerous primary nanoparticles, as seen on the rough surface of the sample. It was also observed that the particle packing was not close-packed but was randomly agglomerated. The size of the primary nanoparticles was too small to conclusively determine the diameter from the SEM images. Energy dispersive spectrum (EDS) analysis indicates that the molar ratio of Pr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn is about 1
Sn is about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, close to the Pr2Sn2O7 stoichiometric composition (Fig. S1†). Transmission electron microscopy (TEM) images further demonstrate the high uniform pellet-like aggregates with a diameters of 20–50 nm, which consist of uniform tiny nanoparticles with a size of several nanometers (Fig. S2† and 2c). More details can be found in the inset of Fig. 2c, which demonstrate that each pellet-like aggregate is composed of abundant randomly assembled nanoparticles with the size of 3–5 nm. Besides, the obvious contrast between the dark parts and the relatively bright parts in this TEM image reveals that the sphere is porous, which endows the Pr2Sn2O7 with high surface area. The typical enlarged lattice-resolved HRTEM image of a nanoparticle (Fig. 2d) shows the clear crystal lattice fringes and the interplane distances are about 2.65 Å, which matches well with the (400) planes of cubic pyrochlore-type Pr2Sn2O7. The bright rings in the selected-area electron diffraction (SAED) pattern of a pellet-like aggregate (the inset of Fig. 2d) can be well indexed to diffraction from the (222), (400), (440) and (444) planes of cubic pyrochlore-type Pr2Sn2O7, which indicates the polycrystalline characteristic assembling nature of nanoparticles.
1, close to the Pr2Sn2O7 stoichiometric composition (Fig. S1†). Transmission electron microscopy (TEM) images further demonstrate the high uniform pellet-like aggregates with a diameters of 20–50 nm, which consist of uniform tiny nanoparticles with a size of several nanometers (Fig. S2† and 2c). More details can be found in the inset of Fig. 2c, which demonstrate that each pellet-like aggregate is composed of abundant randomly assembled nanoparticles with the size of 3–5 nm. Besides, the obvious contrast between the dark parts and the relatively bright parts in this TEM image reveals that the sphere is porous, which endows the Pr2Sn2O7 with high surface area. The typical enlarged lattice-resolved HRTEM image of a nanoparticle (Fig. 2d) shows the clear crystal lattice fringes and the interplane distances are about 2.65 Å, which matches well with the (400) planes of cubic pyrochlore-type Pr2Sn2O7. The bright rings in the selected-area electron diffraction (SAED) pattern of a pellet-like aggregate (the inset of Fig. 2d) can be well indexed to diffraction from the (222), (400), (440) and (444) planes of cubic pyrochlore-type Pr2Sn2O7, which indicates the polycrystalline characteristic assembling nature of nanoparticles.
 | ||
| Fig. 2 (a and b) FE-SEM, (c) TEM, and (d) HRTEM images of the Pr2Sn2O7 crystalline. The inset of (c) shows the TEM image and inset of (d) shows SAED pattern obtained from a nanosphere. | ||
Time-dependent evolution of morphology of Pr2Sn2O7 nanospheres were studied by FE-SEM. Fig. 3 shows typical morphologies of the products obtained at different stages (1, 4, 8 h and 12 h, respectively) of solvothermal reaction at a synthesis temperature of 180 °C. Very small floccus were formed after 1 h of reaction (Fig. 3a). When the reaction was prolonged to 4 h, nanoparticles with relatively spherical shape were appeared (Fig. 3b). The diameter of the clusters was measured to be 10–60 nm. After a reaction time of 8 h, relatively uniform incompact colloidal spheres with rough surface became dominant (Fig. 3c) and there are still some nanoparticles of several nanometers in size. When the reaction time was further prolonged to 12 h, the small Pr2Sn2O7 nanoparticles almost disappeared and the Pr2Sn2O7 nanospheres become more uniform in size and shape.
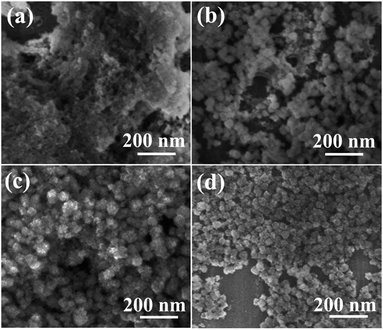 | ||
| Fig. 3 FE-SEM images of Pr2Sn2O7 synthesized at different solvothermal reaction times: (a) 1 h, (b) 4 h, (c) 8 h, (d) 12 h. | ||
The phase and purity of the as-obtained samples with reaction times of 1 h, 4 h, 8 h and 12 h were also determined by XRD, and typical diffraction patterns are shown in Fig. 4. No XRD peaks of Pr2Sn2O7 appear after 1 h of reaction. There is a broad diffraction peak between 20–35° and a small diffraction peak at ∼51°, which could be attributed to tetragonal phase SnO2 (JCPDS, 41-1445) and the sample could be amorphous SnO2. When the reaction was prolonged to 4 h, the diffraction peaks of Pr2Sn2O7 phase are observed, together with some unreacted SnO2. With further prolonged synthesis, the XRD peaks attributed to SnO2 disappear while the XRD peaks of Pr2Sn2O7 become prominent. The XRD patterns of the products prepared for 8, 12, and 24 h (see Fig. 1) seem to be almost the same, except for slightly increasing in XRD intensity with the reaction time being prolonged. When the reaction time was increased from 1 to 24 h, the diffraction peaks became narrower, consistent with the bigger particle sizes.
 | ||
| Fig. 4 XRD patterns of Pr2Sn2O7 synthesized at different solvothermal reaction times: (a) 1 h, (b) 4 h, (c) 8 h, (d) 12 h. | ||
Fig. 5 shows the UV-vis absorption spectrum of Pr2Sn2O7 sample synthesized at different reaction times. A significant blue shift of the main absorption edge in the absorption spectra has been observed as reaction time increased from 1 to 24 h. The absorption threshold of Pr2Sn2O7 samples prepared for 1 h, 4 h and 8 h is located at about 294 nm, 320 nm and 325 nm, respectively, corresponding to a band gap of 3.8–4.2 eV, which is in good agreement with the reported value of SnO2.20–22 In the absorption spectrum, we can observe that the absorption edge of Pr2Sn2O7 prepared for 12 h and 24 h is at around 296 nm (the band gap is about 4.19 eV), this absorption was assigned to the 4f–5d charge transitions of Pr3+ ion.23 It is interesting to point out that there are other several strong absorption peaks in visible light. The strong absorption peaks appearing at around 443 nm (2.80 eV), 470 nm (2.64 eV), 483 nm (2.57 eV) and 582 nm (2.13 eV) are assigned to the 4f–4f transitions of Pr element.23,24
 | ||
| Fig. 5 UV-vis absorption spectrum of the Pr2Sn2O7 samples synthesized at different solvothermal reaction times. | ||
To understand the formation mechanism of such Pr2Sn2O7 nanospheres, different experimental conditions were investigated. The use of pure en as the solvent enables us to obtain the aforementioned Pr2Sn2O7 nanospheres. Interestingly, when 15 mL en was replaced with 14 mL en and 1 mL water, while keeping the other reaction conditions the same, the product were still mainly composed of Pr2Sn2O7 nanospheres with the size of about 10–30 nm as confirmed with XRD pattern and FE-SEM image (Fig. 6a and 7a). When the volume ratio en![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O reaches 2
H2O reaches 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the XRD peaks of prepared Pr2Sn2O7 become narrower and the sample is mainly a mixture of granular aggregates with different sizes (Fig. 6b and 7b). By further increasing the volume of the water, there are some new diffraction peaks at ∼26.6°, ∼33.9°, ∼51.8° appeared, which can be attributed to the (110), (101) and (211) planes of tetragonal phase of SnO2 (JCPDS, 41-1445), respectively. The sample is mainly a mixture of Pr2Sn2O7 and SnO2 particles with different morphologies (Fig. 6c and 7c). With pure water as the solvent, the XRD peaks ascribed to Pr2Sn2O7 disappeared and all the peaks of the product could be readily indexed to tetragonal phase of SnO2 (Fig. 6d). The broadening of the diffraction peaks indicates the crystallite size of this SnO2 sample is small, consistent with the observation of the FE-SEM image (Fig. 7d). As a result, the solvent en plays key and complicated effects on generation of the Pr2Sn2O7 nanospheres structure.
1, the XRD peaks of prepared Pr2Sn2O7 become narrower and the sample is mainly a mixture of granular aggregates with different sizes (Fig. 6b and 7b). By further increasing the volume of the water, there are some new diffraction peaks at ∼26.6°, ∼33.9°, ∼51.8° appeared, which can be attributed to the (110), (101) and (211) planes of tetragonal phase of SnO2 (JCPDS, 41-1445), respectively. The sample is mainly a mixture of Pr2Sn2O7 and SnO2 particles with different morphologies (Fig. 6c and 7c). With pure water as the solvent, the XRD peaks ascribed to Pr2Sn2O7 disappeared and all the peaks of the product could be readily indexed to tetragonal phase of SnO2 (Fig. 6d). The broadening of the diffraction peaks indicates the crystallite size of this SnO2 sample is small, consistent with the observation of the FE-SEM image (Fig. 7d). As a result, the solvent en plays key and complicated effects on generation of the Pr2Sn2O7 nanospheres structure.
En is an excellent strong alkaline solvent which could act as reducing agent and coordinating agent in the formation of many micro/nanostructures.1,25–27 It can adsorb on solid surfaces and control the velocity and direction of crystal growth. In the present case, the en molecules play multiple roles on the formation of Pr2Sn2O7 nanospheres. At the beginning of the reaction, en molecules in the starting solution serve as the coordinating agent using a gauche conformation to chelate with Sn4+ and Pr3+ to form complex ions, such as [Snm(en)n]4+, [Prx(en)y]3+. During the solvothermal process, the complex ions were dissociated to from free Sn4+ ions and Pr3+ ions. There are little water form reactant SnCl4·5H2O, Sn4+ ions are easily hydrolyzed to H2SnO3, which decomposes to amorphous SnO2. Then amorphous SnO2 reacts with free Pr3+ ions in the solution to form Pr2Sn2O7 particles. At the same time, en molecules adsorb on solid surfaces during the reaction to form the Pr2Sn2O7 nanospheres.
We also calculated the band structure of this material. Although the bandgap from Density Functional Theory (DFT) calculations is usually underestimated,28–30 they nonetheless often provide important insight into the physicochemical behavior of the materials investigated. Fig. 8 shows the energy-band dispersion and density of states of Pr2Sn2O7 calculated using Cambridge Sequential Total Energy Package (CASTEP). From this figure we can see that Pr2Sn2O7 is a direct-gap semiconductor material. It is important to note that the bottoms of the conduction bands are mainly composed of hybridized Sn 5s as well as a small quantity of O 2p orbitals. Whereas the tops of the valence bands are composed of hybridized O 2p and Sn 5s orbitals. A minimum band gap between VBM and CBM is about 2.80 eV, indicating of the presence of a semiconducting feature of the materials. The difference of band-gap value obtained by UV-vis spectrum (4.19 eV) and theoretical calculation (2.80 eV) was due to the underestimation by DFT calculation28–30 and size quantization effect of nanomaterial.31
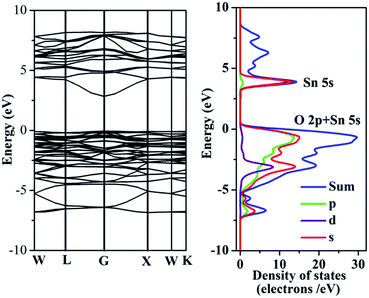 | ||
| Fig. 8 Energy-band diagram and density of states for Pr2Sn2O7 calculated by a density functional method. | ||
Although various properties of the pyrochlores have been investigated in recent years,32–35 to the best of our knowledge, there are no published studies on the application of Pr2Sn2O7. In this study, for the first time, we studied the gas-sensing performances of Pr2Sn2O7 nanospheres and bulk Pr2Sn2O7 to investigate the structural effects on their property. First, the thermal stability of the prepared samples was also evaluated by thermal gravimetric (TG) analysis. TG analysis shows no significant decomposition, and only 2.3% mass loss is observed up to 1000 °C (Fig. S3†), which can be attributed to the desorption of en adsorbed on the surfaces of Pr2Sn2O7 nanospheres. A long plateau is shown in the temperature range of 200–800 °C, indicating high thermal stability of the sample in air. Then bulk Pr2Sn2O7 was obtained by conventional solid-state reaction (SSR) at 1200 °C for 16 h. Finally, the Pr2Sn2O7 gas sensors were prepared. Fig. 9 summarizes the results of Pr2Sn2O7 sensors. Fig. 9a shows the dynamic gas response of the Pr2Sn2O7 to various ethanol concentrations of 100, 200, 400 and 600 ppm at 300 °C. It can be clearly seen that Pr2Sn2O7 nanospheres exhibit better responses to ethanol than SSR-Pr2Sn2O7. Specifically, when Pr2Sn2O7 nanospheres response to ethanol, the response and recovery times of the sensor to 100 ppm ethanol were less than 2 s and 22 s, respectively. As the ethanol concentration increased from 100 ppm to 600 ppm, the response of Pr2Sn2O7 nanospheres and SSR-Pr2Sn2O7 increased from 53.3 and 11.1 to 74.2 and 32.2, respectively. When exposed to 100 ppm ethanol, the responses are about 53.3 and 11.1 for Pr2Sn2O7 nanospheres and SSR Pr2Sn2O7 sample, respectively. In general, the response of semiconducting metal oxide gas sensors was dependent on the operating temperature due to the temperature-dependent gas adsorption and desorption on the oxide surface.36–38 It can be seen from the Fig. 9b that the ethanol response of Pr2Sn2O7 nanospheres increased with the increasing of working temperature and then decreased as the temperature further increases. The Pr2Sn2O7 nanospheres sensor revealed the maximum response at 300 °C. This agrees with the results from other ethanol gas sensors, showing that optimum operating temperatures ranged from 150 to 350 °C.36,37
The repeatability characteristic of Pr2Sn2O7 nanospheres sensor was performed by exposing the sensor to ethanol gas concentration of 100 ppm for three exposure/recovery cycles (Fig. S4†). The presented sensor shows an acceptable repeatability of ethanol gas sensing. It should be emphasized that the morphology of the Pr2Sn2O7 nanospheres remained almost unchanged, except for a slight increase in size after 300 °C heat treatment for 2 h (Fig. S5†), indicating the stability of the Pr2Sn2O7 nanospheres under 300 °C heat treatment. Fig. 9c shows the response of the Pr2Sn2O7 nanospheres sensor towards various testing gases with the concentrations of 100 ppm at the operating temperature of 300 °C. Four kinds of gases have been tested, including C2H5OH, NH3, CO and H2. It is clear from Fig. 9c that the sensor exhibits the highest response towards C2H5OH, which suggests that selective detection of ethanol can be attained.
However the exact sensing mechanism of the Pr2Sn2O7 nanospheres towards ethanol gas need to be further investigated. Most semiconducting oxide gas sensors are based on the conductivity changes caused by the adsorption and desorption of the gas molecules on the surface of the sensing structure.39–41 When the sensing material is exposed to O2 in the atmosphere, the interface of the sensing material adsorbs oxygen and produce oxygen ions with negative charge by capturing electrons from the conduction band. When the sensor is exposed to the ethanol at higher temperature, ethanol reacts with the adsorbed oxygen ions reducing their concentration and result in increasing the semiconductor conductivity. The enhancement of the ethanol gas sensing performance of Pr2Sn2O7 nanospheres can be attributed to the wider band gap (size quantization effect of nanomaterial) and greater sensing surface area due to the nanosphere structure. Fig. 10 shows nitrogen adsorption–desorption isotherms and pore size distribution curves (inset) of the Pr2Sn2O7 nanospheres sample. The sample exhibits a type IV isotherm with an H3 hysteresis loop according to the Brunauer–Deming–Deming–Teller (BDDT) classification, indicating a meso-porous characteristic.42,43 According to the Brunauer–Emmett–Teller (BET) and Langmuir methods, the specific surface areas of Pr2Sn2O7 nanospheres are 89.2 and 124.4 m2 g−1, respectively. The pore size distribution (inset in Fig. 10) shows that Pr2Sn2O7 nanospheres have the pore size distribution in the mesoporous region with a maximum peak pore diameter of 4.4 nm. These results are also consistent with SEM and TEM images results. After 300 °C heat treatment for 2 h, the BET and Langmuir surface areas of Pr2Sn2O7 nanospheres are 72.5 and 100.6 m2 g−1, respectively, which slightly decreased due to the crystal growth after heat treatment. The BET and Langmuir surface areas of the SSR-Pr2Sn2O7 samples are 8.5 and 13.9 m2 g−1, respectively, which explain the better gas-sensing performance of Pr2Sn2O7 nanospheres than that of SSR-Pr2Sn2O7 sample. Nitrogen adsorption–desorption isotherm liner plot of prepared samples are given in the ESI (Tables S1–S3†).
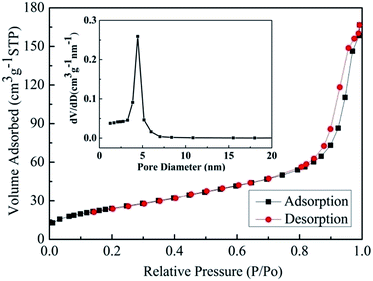 | ||
| Fig. 10 Nitrogen adsorption–desorption isotherms of the prepared Pr2Sn2O7 nanospheres. Inset: the pore size distribution. | ||
Conclusions
In summary, uniform Pr2Sn2O7 nanospheres were successfully synthesized via a simple solvothermal route with ethylenediamine (en) as the solvent. The as-prepared Pr2Sn2O7 nanospheres with the diameters 20–50 nm are composed of nanoparticles with average sizes of 3–5 nm. In our experiments, the solvent en has an important influence in the generation of Pr2Sn2O7 nanospheres. The UV-vis spectrum reveals that the band-gap of synthesized Pr2Sn2O7 nanospheres is about 4.19 eV, much higher than the theoretical calculation value by DFT (2.80 eV). For the first time, the gas-sensing performances of Pr2Sn2O7 nanospheres was studied. Compared to SSR-Pr2Sn2O7 sample, the enhanced gas sensing performances of Pr2Sn2O7 nanospheres could be attributed to the unique mesoporous nanospheres, facilitating the gas diffusion and mass transportation in sensing materials. Moreover, it is anticipated that Pr2Sn2O7 nanospheres could enable potential applications in photocatalytic reaction like some reported lanthanide stannate pyrochlores.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51302001 and 21301002), Anhui Province College Excellent Young Talents Fund (No. 2013SQRL036ZD) and Funds for Distinguished Young Scientists of Anhui Polytechnic University (No. 2015JQ02).Notes and references
- Q. Liu, Y. Zhou, J. Kou, X. Chen, Z. Tian, J. Gao, S. Yan and Z. Zou, J. Am. Chem. Soc., 2010, 132, 14385–14387 CrossRef CAS PubMed
.
- H. Tong, S. Ouyang, Y. Bi, N. Umezawa, M. Oshikiri and J. Ye, Adv. Mater., 2012, 24, 229–251 CrossRef CAS PubMed
.
- X. W. Xie, Y. Li, Z. Q. Liu, M. Haruta and W. J. Shen, Nature, 2009, 458, 746–749 CrossRef CAS PubMed
.
- Q. Liu, W. Zhang, R. Liu and G. Mao, Eur. J. Inorg. Chem., 2015, 2015, 845–851 CrossRef CAS
.
- H. G. Yang and H. C. Zeng, J. Phys. Chem. B, 2004, 108, 3492–3495 CrossRef CAS
.
- J. Zhang, J. Liu, Q. Peng, X. Wang and Y. Li, Chem. Mater., 2006, 18, 867–871 CrossRef CAS
.
- S. Ding, J. S. Chen, G. Qi, X. Duan, Z. Wang, E. P. Giannelis, L. A. Archer and X. W. Lou, J. Am. Chem. Soc., 2011, 133, 21–23 CrossRef CAS PubMed
.
- J. Feng, B. Xiao, Z. X. Qu, R. Zhou and W. Pan, Appl. Phys. Lett., 2011, 99, 201909 CrossRef
.
- K. E. Sickafus, L. Minervini, R. W. Grimes, J. A. Valdez, M. Ishimaru, F. Li, K. J. McClellan and T. Hartmann, Science, 2000, 289, 748–751 CrossRef CAS PubMed
.
- M. Pirzada, R. W. Grimes, L. Minervini, J. F. Maguire and K. E. Sickafus, Solid State Ionics, 2001, 140, 201–208 CrossRef CAS
.
- J. Feng, B. Xiao, R. Zhou and W. Pan, Scr. Mater., 2013, 69, 401–404 CrossRef CAS
.
- J. Lian, K. B. Helean, B. J. Kennedy, L. M. Wang, A. Navrotsky and R. C. Ewing, J. Phys. Chem. B, 2006, 110, 2343–2350 CrossRef CAS PubMed
.
- A. J. Princep, D. Prabhakaran, A. T. Boothroyd and D. T. Adroja, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 104421 CrossRef
.
- A. P. Ramirez, A. Hayashi, R. J. Cava, R. Siddharthan and B. S. Shastry, Nature, 1999, 399, 333–335 CrossRef CAS
.
- H. D. Zhou, C. R. Wiebe, J. A. Janik, L. Balicas, Y. J. Yo, Y. Qiu, J. R. D. Copley and J. S. Gardner, Phys. Rev. Lett., 2008, 101, 227204 CrossRef CAS PubMed
.
- S. Saha, S. Prusty, S. Singh, R. Suryanarayanan, A. Revcolevschi and A. K. Sood, J. Solid State Chem., 2011, 184, 2204–2208 CrossRef CAS
.
- Y. Mao, G. Li, W. Xu and S. Feng, J. Mater. Chem., 2000, 10, 479–482 RSC
.
- J. Zeng, H. Wang, Y. Zhang, M. K. Zhu and H. Yan, J. Phys. Chem. C, 2007, 111, 11879–11887 CAS
.
- W. Wang, S. Liang, J. Bi, J. C. Yu, P. K. Wong and L. Wu, Mater. Res. Bull., 2014, 56, 86–91 CrossRef CAS
.
- W. Wu, S. Zhang, J. Zhou, X. Xiao, F. Ren and C. Jiang, Chem.–Eur. J., 2011, 17, 9708–9719 CrossRef CAS PubMed
.
- Z. Li, Y. Zhou, T. Yu, J. Liu and Z. Zou, CrystEngComm, 2012, 14, 6462–6468 RSC
.
- Ç. Kılıç and A. Zunger, Phys. Rev. Lett., 2002, 88, 095501 CrossRef PubMed
.
- K. Horchani-Naifer and M. Férid, Inorg. Chim. Acta, 2009, 362, 1793–1796 CrossRef CAS
.
- H. Lv, X. Shen, Z. Ji, K. Chen and G. Zhu, New J. Chem., 2014, 38, 2305–2311 RSC
.
- W.-T. Yao and S.-H. Yu, Adv. Funct. Mater., 2008, 18, 3357–3366 CrossRef CAS
.
- Y. Li, H. Liao, Y. Ding, Y. Fan, Y. Zhang and Y. Qian, Inorg. Chem., 1999, 38, 1382–1387 CrossRef CAS
.
- Z. Li, Y. Zhou, J. Song, T. Yu, J. Liu and Z. Zou, J. Mater. Chem. A, 2013, 1, 524–531 CAS
.
- S. Piskunov, E. Heifets, R. I. Eglitis and G. Borstel, Comput. Mater. Sci., 2004, 29, 165–178 CrossRef CAS
.
- C. Di Valentin, G. Pacchioni, A. Selloni, S. Livraghi and E. Giamello, J. Phys. Chem. B, 2005, 109, 11414–11419 CrossRef CAS PubMed
.
- Z. Yi, J. Ye, N. Kikugawa, T. Kako, S. Ouyang, H. Stuart-Williams, H. Yang, J. Cao, W. Luo, Z. Li, Y. Liu and R. L. Withers, Nat. Mater., 2010, 9, 559–564 CrossRef CAS PubMed
.
- S. Baral, A. Fojtik, H. Weller and A. Henglein, J. Am. Chem. Soc., 1986, 108, 375–378 CrossRef CAS PubMed
.
- D. R. Modeshia and R. I. Walton, Chem. Soc. Rev., 2010, 39, 4303–4325 RSC
.
- S. Ikeda, M. Fubuki, Y. K. Takahara and M. Matsumura, Appl. Catal., A, 2006, 300, 186–190 CrossRef CAS
.
- H. Y. Xiao, W. J. Weber, Y. Zhang, X. T. Zu and S. Li, Sci. Rep., 2015, 5, 8265 CrossRef CAS PubMed
.
- R. Perriot and B. P. Uberuaga, J. Mater. Chem. A, 2015, 3, 11554–11565 CAS
.
- H. N. Hieu, N. M. Vuong, H. Jung, D. M. Jang, D. Kim, H. Kim and S.-K. Hong, J. Mater. Chem., 2012, 22, 1127–1134 RSC
.
- G. Sakai, N. Matsunaga, K. Shimanoe and N. Yamazoe, Sens. Actuators, B, 2001, 80, 125–131 CrossRef CAS
.
- A. P. Lee and B. J. Reedy, Sens. Actuators, B, 1999, 60, 35–42 CrossRef CAS
.
- C. D. Gu, H. Zheng, X. L. Wang and J. P. Tu, RSC Adv., 2015, 5, 9143–9153 RSC
.
- D. Zhang, J. Liu, H. Chang, A. Liu and B. Xia, RSC Adv., 2015, 5, 18666–18672 RSC
.
- H. Yan, P. Song, S. Zhang, Z. Yang and Q. Wang, RSC Adv., 2015, 5, 79593–79599 RSC
.
- S. C. Yan, S. X. Ouyang, J. Gao, M. Yang, J. Y. Feng, X. X. Fan, L. J. Wan, Z. S. Li, J. H. Ye, Y. Zhou and Z. G. Zou, Angew. Chem., 2010, 122, 6544–6548 CrossRef
.
- X. W. Lou, D. Deng, J. Y. Lee and L. A. Archer, J. Mater. Chem., 2008, 18, 4397–4401 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra26028k |
| This journal is © The Royal Society of Chemistry 2016 |



