Acid-amplifying microcapsules: preparation, characterization, and application to cationic UV curing†
Abstract
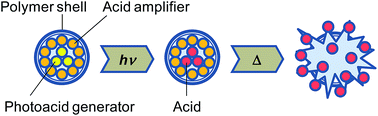
Microcapsules containing photoacid generators and acid amplifiers have been prepared using a liquid-drying method, for the spatial separation of these molecules from resins. The acid-amplifying microcapsules were applied to a cationic UV curing system of an epoxy resin using a 313 nm light source.

 Please wait while we load your content...
Please wait while we load your content...