Enhanced sulfamethoxazole ozonation by noble metal-free catalysis based on magnetic Fe3O4 nanoparticles: catalytic performance and degradation mechanism†
Renli Yina,
Wanqian Guo*a,
Xianjiao Zhoua,
Heshan Zhenga,
Juanshan Dua,
Qinglian Wua,
Joshu Changb and
Nanqi Rena
aState Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology, 73 Huanghe Road, Harbin, Heilongjiang 150090, P. R. China. E-mail: hitgwq@yeah.net
bDepartment of Chemical Engineering, National Cheng Kung University, Tainan, Taiwan. E-mail: changjs@mail.ncku.edu.tw
First published on 1st February 2016
Abstract
In this research, Fe3O4 nanoparticles were prepared by a low-cost route free of other agents, and applied in the catalysis of sulfamethoxazole (SMX) ozonation. It was proven that Fe3O4 nanoparticles significantly enhance SMX ozonation. Using a kinetics analysis, when Fe3O4 particles were added to the ozonation process, the reaction rate constant increased by 51% when the pH was 5. Moreover, we also identified that Fe3O4 enhanced the SMX ozonation removal rate by changing the degradation pathway. It was found that addition of Fe3O4 improved the production of Lewis acid active sites in SMX. These kinds of site in SMX are much easier to attack, which leads to a higher SMX removal rate and lower operational costs for the Fe3O4-based catalytic ozonation process compared to an O3 oxidation process. Finally, the SMX degradation pathways were classified for the first time, based on ozone oxidation types to give a guide for the quick and direct oxidation of SMX and other pollutants.
1 Introduction
Pharmaceutical contamination of environmental samples is considered to present a potential risk for aquatic and terrestrial organisms and is regarded as a rising environmental issue of global concern. Among the various available pharmaceutical compounds, antibiotics have been found in the effluent of a number of sewage treatment plants as well as in surface water and groundwater in the USA, UK, Canada, Germany and China.1–3 Sulfamethoxazole (SMX), a commonly used sulfonamide antibiotic, can be used to treat some of the most frequent illness outbreaks like coccidiosis, diarrhoea and gastroenteritis. Thus, there is large-scale global consumption of SMX in the animal food industry.4 In 2007, SMX was the 6th most universally prescribed antibiotic in Canada.5 In addition, SMX is one of the most commonly used antibiotics in China.6 Due to insufficient pollution control, large amounts of residual SMX has entered into the environment and poses a great threat to human beings. Although in recent years, the pollution control of discharged antibiotics and their by-products have attracted increasing attention from governments and researchers, SMX removal rates are presently low at between 20% and 30% in wastewater treatment plants.7 As a result, untreated SMX residues have caused severe pollution in surface water and underground aquifers in recent years. Furthermore, these residual antibiotics have led to animals and people evolving resistant genes, which could cause antibiotics to final lose function for the treatment of diseases. Therefore, it is necessary to control the discharge concentration of SMX by increasing its removal rate using modified techniques for wastewater treatment processes.Recently, ozone oxidation has proven to be efficient for the removal of refractory pharmaceuticals like sulfonamides and other organic pollutants and for the enhancement of their biodegradability.4,8–14 However, due to their selectivity being limited to the oxidation of organic compounds, the high cost of ozone addition and a poor mass transfer rate in aqueous solution, ozonation processes have not been widely used for practical wastewater treatment. As a consequence, ozone has usually been combined with other technologies, such as ultraviolet/O3, H2O2/O3, ultrasound/O3 and catalyst/O3, in order to improve its utilization rate and save on operation costs.
Modern chemistry and chemical engineering rely heavily on catalytic processes that have dramatically initiated and sustained global industrialization and also played important roles in green chemistry and wastewater treatment. During the past few decades, magnetic Fe3O4 nanoparticles have been the focus of much research because of their considerable properties, such as large surface area to volume ratio, biocompatibility, non-toxicity, simple modification, low cost and recyclability, as well as their high catalytic activity.15 In the field of environmental chemistry, magnetic Fe3O4 nanoparticles are used to catalyze a wide variety of reactions, such as intramolecular C–N cross-coupling reactions, the growth of carbon nanotubes, the decarboxylative cross-ketonisation of aryl- and alkylcarboxylic acids, and heterogeneous Fenton reactions. It has been reported that many Fe3O4-containing composite catalysts, including Au–Fe3O4,16 Pd–Fe3O4,17 Ag@Pd–Fe3O4 (ref. 18) and single crystalline Fe3O4 (ref. 19) have also been shown to exhibit high catalytic activity for the reduction of organic pollutants including pharmaceuticals. However, in all these cases, Fe3O4 is used only as a support for noble metals due to its magnetic properties, but not as a core part of the catalyst. Whether the Fe3O4 nanoparticles themselves have any catalytic activity in the reduction of pharmaceuticals is seldom considered and is still an open question. These catalysts with noble metal coatings have experienced sustainability issues due to their expense, scarcity in nature and deactivation in direct application. In addition, the loss and leaching of toxic metals has caused severe secondary pollution in the environment. Therefore, trialling Fe3O4 nanoparticles free of noble metals and other agents to catalyze pharmaceutical ozonation is economical and essential for the development of green chemistry and environmentally friendly catalysis. Furthermore, the effects of Fe3O4 on catalytic ozonation and its performance should be sufficiently confirmed as well.
Therefore, in this study, the catalytic ozonation of SMX using Fe3O4 nanoparticles as a catalyst was investigated. To clarify the effect of Fe3O4 on SMX degradation, three main aspects were concentrated on: (i) the catalytic performance of Fe3O4, by examining the role Fe3O4 plays in the oxidation system; (ii) the structure and characteristics of Fe3O4, studied by the means of a variety of techniques like TEM, SEM, FTIR, etc.; (iii) the degradation mechanism of SMX, determined by calculating the kinetic constants and speculating on the possible degradation pathways of SMX.
2 Methods and materials
2.1 Preparation of the nanocatalyst
Fe3O4 nanoparticles were prepared by the chemical co-precipitation of Fe3+ and Fe2+ ions in a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.20 Typically, a solution was prepared by adding FeCl2·4H2O (3.68 g) and FeCl3·6H2O (10 g) to deionized H2O (150 mL) under an Ar atmosphere. The resultant solution was left to stir for 30 min at 80 °C. 25 mL of 25% aqueous NH3·H2O was then added dropwise with vigorous stirring to produce a black solid product. The resultant mixture was heated in water bath for 2 h at 60 °C and the black magnetite solid product was then washed with deionized H2O, filtered and dried in an oven at 105 °C for 24 h.
1.20 Typically, a solution was prepared by adding FeCl2·4H2O (3.68 g) and FeCl3·6H2O (10 g) to deionized H2O (150 mL) under an Ar atmosphere. The resultant solution was left to stir for 30 min at 80 °C. 25 mL of 25% aqueous NH3·H2O was then added dropwise with vigorous stirring to produce a black solid product. The resultant mixture was heated in water bath for 2 h at 60 °C and the black magnetite solid product was then washed with deionized H2O, filtered and dried in an oven at 105 °C for 24 h.
2.2 Analytical methods
Sulfamethoxazole (C10H11N3O3S, analytical reagent grade, 99.0%) was purchased from Sigma and used as received without further purification. The SMX concentration was determined by UPLC (Waters, C18, λ = 265 nm). The mobile phase was a mixture of acetonitrile and 0.1% formic acid with a volume ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70. A 2 μL volume was injected using an auto sampler. The intermediates of SMX degradation were measured using a HPLC-MS/MS (Finnigan, LCQ-DECA-XP-MAX) instrument, which was equipped with a Zorbax SB-C18 HPLC column (150 mm × 2.1 mm × 3.5 μm, Agilent). In the MS analysis, the ionization for the MS was operated in APCI mode with the negative ion mode. In this paper, the method applied for the kinetics was based on a reaction comparison between the degradation rate of SMX and that of a reference compound. Fumaric acid (FA) was chosen to be the reference compound for the comparison reaction (details shown in ESI†).
70. A 2 μL volume was injected using an auto sampler. The intermediates of SMX degradation were measured using a HPLC-MS/MS (Finnigan, LCQ-DECA-XP-MAX) instrument, which was equipped with a Zorbax SB-C18 HPLC column (150 mm × 2.1 mm × 3.5 μm, Agilent). In the MS analysis, the ionization for the MS was operated in APCI mode with the negative ion mode. In this paper, the method applied for the kinetics was based on a reaction comparison between the degradation rate of SMX and that of a reference compound. Fumaric acid (FA) was chosen to be the reference compound for the comparison reaction (details shown in ESI†).
The powder XRD pattern for the Fe3O4 catalyst was recorded with a Bruker D8 advance diffractometer. A transmission electron microscope (TEM, Hitachi H-7650) with an accelerating voltage of 100 kV, was used to observe the morphology of the catalyst. Fourier transform infrared (FTIR) spectrometry was carried out on a Spectrum One spectrometer in order to obtain information about the valence of the catalyst.
2.3 Catalytic tests
The degradation experiments were carried out in a conical flask (containing 200 mL of reaction solution) at 25 °C. The dosage of catalyst was 1.0 g L−1 and the concentration of SMX was 50 mg L−1 (≈0.2 mmol L−1). All of the experiments were carried out under constant stirring to make sure the catalyst was well dispersed. H3PO4 and NaOH were used to adjust the pH of the solution. Before adding O3, the suspension containing the catalyst and SMX was stirred for 0.5 h in order to achieve adsorption equilibrium. The degradation reaction was then initiated by introducing O3 (2 g h−1) into the SMX solution. At a given interval of degradation, the concentration of a sample was determined by UPLC. After adsorption and degradation the magnetite was collected and dried in a vacuum oven at 105 °C for 24 h.3 Results and discussion
3.1 Catalytic performance
To understand the role of Fe3O4 in the SMX ozonation removal process, various sets of experiments were conducted under different operating parameters, including ozonation only, ozonation/Fe3O4, and adsorption of SMX onto Fe3O4.As shown in Fig. 1, it was observed that the SMX concentrations decreased with increasing reaction time during the two processes; ozonation only and ozonation/Fe3O4. After ozonation for 5 min, compared with the ozonation only process, which had a SMX degradation efficiency of 85%, the SMX degradation efficiency had reached 97% in the presence of the Fe3O4 catalyst. The experimental results showed that the SMX removal followed apparent pseudo-first-order kinetics, (d[SMX])/dt = k[SMX], where [SMX] is the SMX concentration at time t and k is the pseudo-first-order rate constant. More importantly, the pseudo-first-order rate constant of the catalytic SMX ozonation with Fe3O4 was 0.61 min−1, which was 1.6 times higher than that of the ozonation only (0.38 min−1). In this study, the Fe3O4 improved the SMX pseudo-first-order rate constant by 60%, while Hou et al.21 found that their Fe3O4 catalyst enhanced the SMX pseudo-first-order constant by 38% with the observed pseudo-first-order constant changing from 0.18 min−1 to 0.25 min−1 ([SMX] = 50 mg L−1; pH = 7.0; [Fe3O4] = 0.3 g L−1) in 20 min. Meanwhile, Gonçalves et al.22,23 found that multi-walled carbon nanotubes could provide a 25–50% increase in SMX ozonation after 3 h, which indicates that in the current study, Fe3O4 has much better catalytic performance. Ozone has a strong affinity towards Lewis acid sites and the Fe3O4 nanoparticles can offer more Lewis acid sites on their surfaces, enhancing the redox process involved in ozone adsorption/decomposition.24,25 Therefore, in this study, the ozone decomposition rate was enhanced in the catalyst suspensions, and the removal rate was consequently improved.
In order to determine the enhancement mechanism of the SMX degradation, SMX adsorption experiments on the catalyst surface were conducted under the same experimental conditions as used in the catalytic activity tests, but in the absence of ozone. Although a small amount of SMX adsorption onto the Fe3O4 catalyst was observed (data not shown), this adsorption was too weak to affect the degradation efficiency in the Fe3O4 catalytic ozonation process. However, the degradation rate and the observed rate constant were indeed enhanced in the catalytic process. Consequently, the Fe3O4 nanoparticles certainly demonstrated catalytic effects on the SMX ozonation process rather than adsorption. In order to reveal how the Fe3O4 nanoparticles catalyze the degradation of SMX, the characterization of the catalyst is analysed in the next section.
3.2 Characterization of the Fe3O4 catalyst
The surface chemistry of the Fe3O4 nanoparticles was studied using FTIR spectroscopy. In the spectrum show in Fig. S1,† the set of broad peaks centered at 574 cm−1 can be ascribed to stretching vibrations of the Fe–O bonds in Fe3O4 (ref. 26) and the presence of vibration bands at 3400 (O–H stretching) demonstrate the successful synthesis of Fe3O4 nanoparticles. The XRD pattern of the Fe3O4 nanoparticles is shown in Fig. S2.† Diffraction peaks are seen at 2θ = 30.2°, 35.6°, 42.3°, 53.6°, 57.1° and 62.6°, which can be indexed to the cubic-phase of Fe3O4, which is in agreement with the literature27 and corresponds well to the standard card for magnetite (PDF no. 65-3107). TEM and SEM images demonstrated that the Fe3O4 nanoparticles were quasi-spherical in shape, and had a nearly uniform distribution of particle size (3–5 nm, Fig. S3 and S4†). An EDS (energy dispersive X-ray fluorescence spectrometer) coupled with a scanning electron microscope (SEM) was used for microanalysis on single spots or on small areas using an accelerating voltage of 20 kV. Elemental SEM/EDS analysis shows the presence of iron and oxygen, suggesting that the Fe salt is pure. The results showing elements in the SEM/EDS sample as given by the experimental engineer are shown in Table S1.†3.3 The degradation mechanism of SMX
| pH | Ratio kSMX/kFA | kFA | kSMX | Ratio kSMX(Fe3O4+O3)/kSMX(O3) | kSMX(Fe3O4+O3) |
|---|---|---|---|---|---|
| a kFA: ozonation rate of FA; kSMX: ozonation rate of SMX; kSMX(Fe3O4+O3): oxidation rate of SMX in the catalytic system. | |||||
| 5 | 1.35 | 1 × 105 | 1.35 × 105 | 1.51 | 2.04 × 105 |
| 7 | 2.58 | 1.5 × 105 | 3.87 × 105 | 1.34 | 5.19 × 105 |
The SMX degradation curves indicate that the SMX degradation reaction follows a pseudo-first-order kinetic model under the experimental conditions (both O3 and catalytic ozonation), as shown in Fig. 1. It can also be seen that the ozone oxidation SMX degradation reaction fits this kinetic model well. However, in the catalytic ozonation system, it shows a clear deviation from the fitting curve. As intermediate accumulation occurs with access to the Fe3O4 catalyst, the degradation of the parent SMX molecules is initially affected, making the degradation rate slower than the theoretical value. The rate then becomes higher than the theoretical value with prolonged reaction time due to SMX and its intermediates being gradually degraded, indicating that the Fe3O4 nanoparticles not only catalyze SMX degradation and enhance the oxidation of its intermediates, but it also intensifies competition between SMX and its intermediates for O3 contact to achieve a higher pollutant removal rate. Therefore, it could be reasonably assumed that the Fe3O4 nanocatalyst had an influence on the SMX ozonation degradation, its intermediates (either species or quantity) and pathways. For deeper understanding of the role that Fe3O4 plays in this process, the possible SMX degradation pathways needed to be clarified.
 | ||
| Fig. 2 Total ion chromatographs and mass spectra for the O3-only and Fe3O4 catalytic ozonation processes. | ||
| m/z | Structure | O3 | O3 + Fe3O4 |
|---|---|---|---|
| a D: fragmentation detected; ND: fragmentation not detected. | |||
| 97 |  |
D | D |
| 107 | 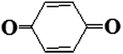 |
D | D |
| 113 |  |
D | D |
| 115 | 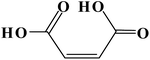 |
D | D |
| 156 |  |
D | D |
| 179 |  |
D | D |
| 226 | 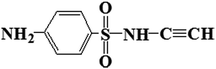 |
D | D |
| 268 |  |
D | D |
| 282a |  |
D | D |
| 282b |  |
D | D |
| 298 |  |
ND | D |
| 348 |  |
ND | D |
The enhancement mechanism of the nanocatalyst was also identified. Although the direct and indirect types of ozone oxidation were both observed in the O3-only and Fe3O4 catalytic ozonation processes, it was found that the introduction of Fe3O4 to the ozonation improved the production of Lewis acid active sites, which attracted greater attention from O3 bubbles, so making SMX easier to attack and resulting in a higher SMX removal rate, shorter reaction time, smaller ozone input and lower operational costs for the catalytic ozonation process. Furthermore, degradation pathways of SMX were classified along with the different ozone oxidation types. Once the intermediates with lower toxicity and fewer hazards are determined, we could regulate the degradation of SMX-like pollutants directly and quickly towards these known intermediates by changing the operational conditions to control the ozone oxidation type, which will have benefits for targeted SMX-like pollutant removal.
4 Conclusions
Fe3O4 nanoparticles free of any noble metals or other agents have proven to be an effective method to facilitate and enhance SMX ozonation in water, with evidence that they change the degradation pathway of SMX. Calculation of the kinetic constants also demonstrated that the SMX degradation rate was improved with the aide of Fe3O4. At the same time, new insights into SMX ozonation degradation pathways were proposed for the first time, according to the ozone oxidation type, which gives guidelines for the direct degradation of SMX and other pollutants to less toxic and less harmful products by controlling the operational conditions. In summary, this reusable, stable and O3 and Fe3O4-based nanocatalyst could be used to remove pharmaceuticals and organic pollutants, which is significant for the development of advanced technologies for environmental remediation from both an economic and environmental point of view.Acknowledgements
This work was financially supported by the Harbin Institute of Technology Fund for young top-notch talent teachers (AUGA5710052514). The authors also gratefully acknowledge financial support from the National Key Technology Support Program (2014BAD02B03), the Department of Education Fund for Doctoral Tutors (20122302110054), the State Key Laboratory of Urban Water Resource and Environment (2014TS06), and the Postdoctoral Science-Research Foundation of Heilongjiang Province (LBH-Q12106).References
- K. Kummerer, Chemosphere, 2009, 75, 417–434 CrossRef PubMed.
- K. Kummerer, Chemosphere, 2009, 75, 435–441 CrossRef PubMed.
- P. Verlicchi, M. Al Aukidy and E. Zambello, Sci. Total Environ., 2012, 429, 123–155 CrossRef CAS PubMed.
- R. F. Dantas, S. Contreras, C. Sans and S. Esplugas, J. Hazard. Mater., 2008, 150, 790–794 CrossRef CAS PubMed.
- D. Nasuhoglu, V. Yargeau and D. Berk, J. Hazard. Mater., 2011, 186, 67–75 CrossRef CAS PubMed.
- H. W. Leung, T. B. Minh, M. B. Murphy, J. C. Lam, M. K. So, M. Martin, P. K. Lam and B. J. Richardson, Environ. Int., 2012, 42, 1–9 CrossRef CAS PubMed.
- X. Liu, T. Garoma, Z. Chen, L. Wang and Y. Wu, Chemosphere, 2012, 87, 1134–1140 CrossRef CAS PubMed.
- G. Ji, B. Zhang and Y. Wu, J. Hazard. Mater., 2012, 225–226, 1–7 CrossRef CAS PubMed.
- P. C. Sangave, P. R. Gogate and A. B. Pandit, Chemosphere, 2007, 68, 42–50 CrossRef CAS PubMed.
- P. R. Gogate, S. Mededovic-Thagard, D. McGuire, G. Chapas, J. Blackmon and R. Cathey, Ultrason. Sonochem., 2014, 21, 590–598 CrossRef CAS PubMed.
- L. Zhao, W. Ma, J. Ma, J. Yang, G. Wen and Z. Sun, Ultrason. Sonochem., 2014, 21, 104–112 CrossRef CAS PubMed.
- I. Arslan-Alaton and A. E. Caglayan, Chemosphere, 2005, 59, 31–39 CrossRef CAS PubMed.
- N. Le-Minh, S. J. Khan, J. E. Drewes and R. M. Stuetz, Water Res., 2010, 44, 4295–4323 CrossRef CAS PubMed.
- K. K. Jyoti and A. B. Pandit, Biochem. Eng. J., 2004, 18, 9–19 CrossRef CAS.
- V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J. M. Basset, Chem. Rev., 2011, 111, 3036–3075 CrossRef CAS PubMed.
- F.-H. Lin and R.-A. Doong, J. Phys. Chem. C, 2011, 115, 6591–6598 CAS.
- H.-Q. Wang, X. Wei, K.-X. Wang and J.-S. Chen, Dalton Trans., 2012, 41, 3204–3208 RSC.
- K. Jiang, H.-X. Zhang, Y.-Y. Yang, R. Mothes, H. Lang and W.-B. Cai, Chem. Commun., 2011, 47, 11924–11926 RSC.
- H. Li, J. Liao and X. Zhang, J. Mater. Chem. A, 2014, 2, 17530 CAS.
- C.-H. Liu, Y.-H. Chuang, T.-Y. Chen, Y. Tian, H. Li, M.-K. Wang and W. Zhang, Environ. Sci. Technol., 2015, 49, 7726–7734 CrossRef CAS PubMed.
- L. Hou, H. Zhang, L. Wang, L. Chen, Y. Xiong and X. Xue, Sep. Purif. Technol., 2013, 117, 46–52 CrossRef CAS.
- A. G. Gonçalves, J. J. M. Órfão and M. F. R. Pereira, Catal. Commun., 2013, 35, 82–87 CrossRef.
- A. G. Goncalves, J. J. Orfao and M. F. Pereira, J. Hazard. Mater., 2012, 239–240, 167–174 CrossRef CAS PubMed.
- W. Li and S. T. Oyama, J. Am. Chem. Soc., 1998, 120, 9047–9052 CrossRef CAS.
- A. Lv, C. Hu, Y. Nie and J. Qu, Appl. Catal., B, 2012, 117–118, 246–252 CrossRef CAS.
- X. Liang, S. Zhu, Y. Zhong, J. Zhu, P. Yuan, H. He and J. Zhang, Appl. Catal., B, 2010, 97, 151–159 CrossRef CAS.
- H. Niu, D. Zhang, S. Zhang, X. Zhang, Z. Meng and Y. Cai, J. Hazard. Mater., 2011, 190, 559–565 CrossRef CAS PubMed.
- W. Q. Guo, R. L. Yin, X. J. Zhou, J. S. Du, H. O. Cao, S. S. Yang and N. Q. Ren, Ultrason. Sonochem., 2015, 22, 182–187 CrossRef CAS PubMed.
- A. Lv, C. Hu, Y. Nie and J. Qu, Appl. Catal., B, 2010, 100, 62–67 CrossRef CAS.
- M. Gomez-Ramos Mdel, M. Mezcua, A. Aguera, A. R. Fernandez-Alba, S. Gonzalo, A. Rodriguez and R. Rosal, J. Hazard. Mater., 2011, 192, 18–25 Search PubMed.
- R. Andreozzi, M. Canterino, R. Marotta and N. Paxeus, J. Hazard. Mater., 2005, 122, 243–250 CrossRef CAS PubMed.
- R. F. Dantas, M. Canterino, R. Marotta, C. Sans, S. Esplugas and R. Andreozzi, Water Res., 2007, 41, 2525–2532 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra25994k |
| This journal is © The Royal Society of Chemistry 2016 |


