Immobilized palladium nanoparticles within polymers as active catalysts for Suzuki–Miyaura reaction†
Ting Chen,
Fei Mao,
Zhengliang Qi,
Yan Li,
Rizhi Chen,
Yong Wang and
Jun Huang*
State Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemical Engineering, Nanjing Tech University, Nanjing 210009, China. E-mail: junhuang@njtech.edu.cn; Fax: +86 25-83172261
First published on 1st February 2016
Abstract
A highly active and reusable catalyst Pd@PNP was developed for Suzuki–Miyaura reaction of aryl chlorides and bromides with aryl boronic acids, and the corresponding biphenyl compounds were obtained in good to excellent yields. Triphenylphosphine and palladium nanoparticles were immobilized in situ in the polymer formed from Pd catalyzed coupling of tris(4-bromophenyl)amine and benzene-1,4-diboronic acid. The immobilized triphenylphosphine enhanced the activity and the stability of the catalyst Pd@PNP, and the catalyst Pd@PNP can be reused at least 5 times with good activity. Functional groups, such as methoxyl, nitrile, tert-butyl, nitro, acyl and formyl groups, were well tolerated under the reaction conditions, and the corresponding products were obtained in high yields.
Introduction
Palladium catalyzed cross-coupling reactions are applied widely for synthesis of pharmaceuticals,1 agricultural chemicals and natural products.2 Although some highly active ligand/palladium systems were designed and used for Pd catalyzed Suzuki–Miyaura reactions,3 Heck reactions,4 Sonogashira reactions and Negishi reactions,5 heterogeneous Pd catalyst systems are more convenient for the cross-coupling reactions in practical applications, as Pd/ligand systems are difficult to recycle, and the Pd/ligand may be leaked into the products. The recovery of the active Pd/ligand catalyst systems is highly valuable not only for economic reasons but also for avoiding product contamination. Therefore, active heterogeneous Pd catalysts were designed and applied for cross-coupling reactions, including Pd supported on carbon,6 graphite,7 metal oxides,8 hybrid materials and polymers.9 But heterogeneous Pd catalysts are usually less active than Pd/ligand systems, especially for activation of aryl chlorides. Aryl chlorides are cheaper than aryl bromides and iodides and commercially available widely, but the activation of aryl chlorides is difficult as the C–Cl bond is much stronger than C–Br and C–I bonds. Thus, it is highly desirable to develop highly active heterogeneous catalysts for the cross coupling reactions.Recently, we reported highly active Pd heterogeneous catalysts for Suzuki–Miyaura reaction, and the catalysts can be reused several times and no P/Pd contamination was found in the products.10 The electron rich and bulky P ligands were anchored into the Pd catalyzed polyphenylene polymers, and the active Pd nanoparticles were in situ formed and trapped into the polymers as highly active Pd heterogeneous catalysts. Moreover, Pd nanoparticles supported in ionic solid polymers were reported as highly active heterogeneous catalysts for Suzuki–Miyaura reaction, and only 10 ppm of Pd catalyst was required for Suzuki–Miyaura reaction of aryl bromides.11 In addition, we also reported the C–O coupling and C–CN coupling of aryl chlorides and bromides with heterogeneous or homogeneous catalyst systems.12
Here, the in situ formed Pd nanoparticles were trapped and immobilized into the polymers with triphenylphosphine ligand giving highly active Pd heterogeneous catalysts for Suzuki–Miyaura reaction. The Pd@PNP catalysts were found to be highly active for the Suzuki–Miyaura coupling of the aryl bromides and chlorides with aryl boronic acids. As the P ligands and Pd were both immobilized into the polymers, the biphenyl products can be separated easily without contamination from P and Pd. Additionally, the Pd@PNP catalysts are stable in air since the TPP ligand is not sensitive to air.
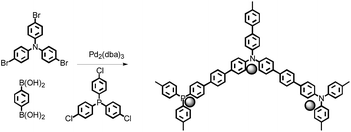
The Pd@PNP catalysts were synthesized by palladium catalyzed Suzuki–Miyaura reaction of tris(4-bromophenyl) amine with benzene-1,4-diboronic acid. Tris(dibenzylideneacetone)-dipalladium with tris(4-chlorophenyl)phosphine was selected as the Pd catalyst, and then the ligand tris(4-chlorophenyl)phosphine could participate in the Suzuki–Miyaura reaction of tris(4-bromophenyl)amine with benzene-1,4-diboronic acid to form the polymers. Moreover, Pd nanoparticles were trapped and immobilized into polymers to form the Pd@PNP catalysts (see Scheme 1).
Experimental section
Preparation of Pd@PNP catalysts
The procedure for the synthesis of the Pd@PNP catalyst was as follows. 0.482 g (1.0 mmol) of tris(4-bromophenyl)amine, 0.348 g (2.1 mmol) of benzene-1,4-diboronic acid, 0.0458 g (0.05 mmol) of Pd2(dba)3, 0.146 g (0.4 mmol) of tris(4-chlorophenyl)phosphine and 0.4119 g (3.0 mmol) of K2CO3 were added into a Schlenk tube with 30 mL of DMF under argon. And then the tube was heated to 150 °C in an oil bath with stirring for 24 h. After the reaction mixture cooled to room temperature, the precipitated yellow powder was collected by centrifugation. After washing with water and DMF 3 times, the powder was dried under vacuum and stored in an argon atmosphere as the Pd@PNP catalyst. The amount of Pd in the Pd@PNP catalyst was found to be 1.81 wt% (determined by ICP-AES).The Pd@PNP-1 was prepared similarly with 0.0229 g (0.025 mmol) of Pd2(dba)3 and 0.073 g (0.2 mmol) of tris(4-chlorophenyl)phosphine added. The amount of Pd in the Pd@PNP-1 catalyst was found to be 0.92 wt% (determined by ICP-AES).
The Pd@PNP-2 was synthesized with similar procedures using 0.0224 g (0.1 mmol) of Pd(OAc)2 instead of Pd2(dba)3. The amount of Pd in the Pd@PNP-2 catalyst was found to be 1.91 wt% (determined by ICP-AES).
The Pd@PN was synthesized using similar procedures using tetrakis(triphenylphosphine)palladium instead of Pd2(dba)3 without the ligand tris(4-chlorophenyl)phosphine. 0.482 g (1.0 mmol) of tris(4-bromophenyl)amine, 0.2486 g (1.5 mmol) of benzene-1,4-diboronic acid, 0.1156 g (0.1 mmol) of tetrakis(triphenylphosphine)palladium and 0.2746 g (2.0 mmol) of K2CO3 were added into a Schlenk tube with 30 mL of DMF under argon. The amount of Pd in the Pd@PN catalyst was found to be 1.92 wt% (determined by ICP-AES).
Typical procedure for Pd catalyzed Suzuki–Miyaura coupling reaction of aryl halides with aryl boronic acid
Aryl chlorides (1.0 mmol), phenylboronic acid (1.2 mmol), Pd@PNP catalyst (0.2–0.3% Pd), K2CO3 (2 mmol) and methanol (2.0 mL) were added into a pressure tube under Ar. The pressure tube was placed in an oil bath for 14 h at 80 °C. The reaction mixture was cooled to room temperature and the crude products were purified in a column with silica gel (eluting with ethyl acetate/hexane).The coupling reaction of aryl bromides with aryl boronic acids was performed similarly, except with the addition of a lower amount of catalyst Pd@PNP. The crude products were purified in a column with silica gel (eluting with ethyl acetate/hexane).
Reusability of catalyst Pd@PNP
The coupling of 4-chlorotoluene with phenylboronic acid was used to test the reusability of catalyst Pd@PNP. After centrifugation and washing with ethyl acetate, the catalyst Pd@PNP was added to the reaction mixture again for the coupling of 4-chlorotoluene with phenylboronic acid, and the yield of methyl biphenyl was determined by GC.Results and discussions
Catalyst characterization
To study the structure of the catalyst, the Pd@PNP was characterized by TEM (transmission electron microscope) and SEM (scanning electron microscopy) presented in Fig. 1 and S1,† respectively. From Fig. 1, we can see that Pd nanoparticles were dispersed on the PNP polymers, and the diameter size of the Pd particles is about 1–5 nm (Fig. 1, left). The Pd nanoparticles were retained well and the average diameter of the Pd particles was about 1–5 nm too, and nearly no evident aggregation was found in the recycled Pd@PNP catalyst after 5 reaction runs (Fig. 1, right). The SEM image of the Pd@PNP showed that the catalyst Pd@PNP contained irregularly shaped particles with irregular pores (Fig. S1†). From nitrogen adsorption–desorption analysis, the BET surface area of the catalyst Pd@PNP was about 48 m2 g−1 (Fig. S2†). The average pore diameter of the catalyst Pd@PNP was about 6.7 nm. The thermogravimetric analysis (TGA) showed that the Pd@PNP was thermally stable up to 500 °C. As shown in the TG curve (Fig. S3†), a sharp weight loss from room temperature to 150 °C occurred due to the loss of moisture and solvent (DMF). There is no evident mass loss between 150 °C and 500 °C, which showed good thermostability of the catalyst Pd@PNP. The catalyst Pd@PNP decomposed slowly after 500 °C. | ||
| Fig. 1 TEM images of Pd@PNP catalyst (left); the catalyst after five reaction runs (right); scale bar, 10 nm. | ||
The X-ray diffraction patterns of Pd@PNP (Fig. S4†) exhibited broad peaks at 2θ = 40.016°, 46.664°, 68.378° and 82.199° corresponding to Pd metal diffraction lines (111), (200), (220) and (311) respectively. The diffraction peaks in this pattern confirm that Pd was supported on solid N containing polymer. The EDX elemental analyses of the Pd@PNP revealed the presence of C, N, P, Br and Pd (Fig. S5†). No Cl peak was found, which may be because the amount of tris(4-chlorophenyl)phosphine added was low, and most of the Cl was removed via the reaction with benzene-1,4-diboronic acid. The existence of P was in agreement with this deduction. The FT-IR spectra of the Pd@PNP catalyst is shown in Fig. S6.† The IR bands between 1500 cm−1 and 500 cm−1 are for the phenyl rings.
Suzuki–Miyaura coupling of 4-chlorotoluene with phenylboronic acid
The catalyst Pd@PNP was studied for the Suzuki–Miyaura coupling of 4-chlorotoluene with phenylboronic acid, and reaction conditions were optimized. The catalysts Pd@PNP, Pd@PNP-1, Pd@PNP-2 and Pd@PN were tested for comparison, and the catalyst Pd@PNP was found to be more active than the other catalysts for the coupling reaction (Table 1, entries 1–4). The formation of the polymers was affected by the amount of Pd added and the Pd source, as the polymerization was catalyzed by the active Pd nanoparticles. Comparing Pd@PNP with Pd@PNP-1, the decrease of added Pd2(dba)3 may slow down the formation of polymers, and simultaneously lead to the aggregation of Pd nanoparticles. In addition, the added Pd(Ac)2 needed to be reduced to active Pd(0) by benzene-1,4-diboronic acid first, which slowed down the formation of the polymers and led to the aggregation of Pd nanoparticles. In Pd@PN, Pd[P(C6H5)3]4 was used as a catalyst and Pd source. As the P(C6H5)3 cannot couple with benzene-1,4-diboronic acid, and Pd nanoparticles in the polymers were not stable enough, the aggregated Pd particles were not quite active for the coupling reaction. The solvents DMF, DMSO, toluene, ethanol, 1,4-dioxane and methanol were used, and methanol was found to be the best solvent for the coupling reaction (Table 1, entries 5–10). The bases Na2CO3, K3PO4, K2CO3, KOH and NaOH were tested, and K2CO3 was the most suitable base for the coupling reaction (Table 1, entries 11–13). Then, the added Pd amount and reaction time were optimized, and 4-phenyltoluene was obtained in 99% yield with 0.2 mol% of Pd in 14 hours (Table 1, entries 14–17). The Pd@PNP stored in air for 1 week was used for the coupling of 4-chloroluente with phenylboronic acid, and 4-phenyltoluene was obtained in 99% yield also, which showed the Pd@PNP was not sensitive to air (Table 1, entry 18).| Entry | Catalyst | Base | Solvent | Pd (mol%) | Yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 4-chlorotoluene, 1.0 mmol; phenylboronic acid, 1.2 mmol; base, K2CO3, 2 mmol; at 80 °C; for 16 h; under argon.b K3PO4, 1.3 mmol; at 100 °C.c Na2CO3, 2.0 mmol; NaOH and KOH, 4.0 mmol.d At 70 °C.e 14 h.f 12 h.g The catalyst Pd@PNP was exposed to the air for a week. | |||||
| 1a | Pd@PNP | K2CO3 | Methanol | 0.1 | 63 |
| 2a | Pd@PNP-1 | K2CO3 | Methanol | 0.1 | 29 |
| 3a | Pd@PNP-2 | K2CO3 | Methanol | 0.1 | 4 |
| 4a | Pd@PN | K2CO3 | Methanol | 0.1 | 5 |
| 5b | Pd@PNP | K3PO4 | DMF | 0.1 | 36 |
| 6b | Pd@PNP | K3PO4 | DMSO | 0.1 | <1 |
| 7b | Pd@PNP | K3PO4 | Ethanol | 0.1 | 15 |
| 8b | Pd@PNP | K3PO4 | Toluene | 0.1 | 2 |
| 9b | Pd@PNP | K3PO4 | 1,4-Dioxane | 0.1 | 28 |
| 10 | Pd@PNP | K3PO4 | Methanol | 0.1 | 38 |
| 11c | Pd@PNP | Na2CO3 | Methanol | 0.1 | 61 |
| 12c | Pd@PNP | KOH | Methanol | 0.1 | 15 |
| 13c | Pd@PNP | NaOH | Methanol | 0.1 | 11 |
| 14 | Pd@PNP | K2CO3 | Methanol | 0.2 | 99 |
| 15d | Pd@PNP | K2CO3 | Methanol | 0.2 | 86 |
| 16e | Pd@PNP | K2CO3 | Methanol | 0.2 | 99 |
| 17f | Pd@PNP | K2CO3 | Methanol | 0.2 | 95 |
| 18g | Pd@PNP | K2CO3 | Methanol | 0.2 | 99 |
Suzuki–Miyaura coupling of aryl chlorides with aryl boronic acids
With optimized reaction conditions, the catalyst Pd@PNP was tested for Suzuki–Miyaura coupling reaction of aryl chlorides with aryl boronic acids, and the results are presented in Table 2. The cross coupling of aryl chlorides with both electron withdrawing substituents and electron donating substituents afforded the corresponding biphenyl compounds in good to excellent yields (Table 2, entries 1–8). The acyl, formyl, nitro and nitrile groups were tolerated well in the reaction conditions, and high yields of the corresponding products were obtained (Table 2, entries 1–6). The coupling of deactivated aryl chlorides, such as 4-chlorotoluene and 2-chloroanisole, gave the corresponding products in high yields also (Table 2, entries 7 and 8). Moreover, phenylboronic acids with functional groups, including methyl, methoxyl and nitrile groups, were coupled well with chlorobenzene, and the corresponding biphenyl products were obtained in 83% to 96% yields (Table 2, entries 9–11).| Entry | R1 | R2 | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: aryl chlorides, 1.0 mmol; phenylboronic acid 1.2 mmol; Pd catalyst Pd@PNP, Pd, 0.2 mol%; K2CO3, 2 mmol; methanol, 2 mL; at 80 °C; 14 h; under argon.b Isolated yield.c Pd, 0.3 mol%. | |||
| 1c | 4-MeO | H | 96 |
| 2 | 4-MeCO | H | 96 |
| 3 | 4-CHO | H | 94 |
| 4 | 4-NO2 | H | 93 |
| 5 | 2-CN | H | 70 |
| 6c | 2-NO2 | H | 94 |
| 7c | 2-Me | H | 95 |
| 8c | 2-MeO | H | 95 |
| 9c | H | 4-CN | 83 |
| 10c | H | 4-CH3 | 95 |
| 11c | H | 4-MeO | 96 |
Suzuki–Miyaura coupling of aryl bromides with phenylboronic acid
Aryl bromides were also examined for the Suzuki–Miyaura coupling with phenylboronic acid, and the results are listed in Table 3. As the C–Br bond (in aryl bromides) is weaker than the C–Cl bond, Suzuki–Miyaura coupling of aryl bromides with phenylboronic acid was performed with lower catalyst loading (catalyst Pd@PNP, 0.02–0.05 mol% of Pd added). The Pd catalyst Pd@PNP showed high efficiency for the cross coupling reaction of aryl bromides with phenylboronic acid. The coupling of aryl bromides with electron withdrawing or electron donating groups gave the corresponding products in good to excellent yields. The para-substituents in aryl bromides, including methoxyl, nitrile, tert-butyl, acyl and formyl groups, were well tolerated under the reaction conditions, and the corresponding products were obtained in high yields (Table 3, entries 1–5). The coupling reaction of ortho-substituted and 2,6-dimethyl substituted bromobenzene afforded the corresponding products in good yields also (Table 3, entries 6–10).| Entry | R | Yieldb (%) |
|---|---|---|
| a Reaction conditions: aryl bromides, 5.0 mmol; phenylboronic acid, 6.0 mmol; Pd@PNP Pd catalyst, Pd, 0.02 mol%; K2CO3, 10.0 mmol; methanol, 3 mL; at 70 °C; 11 h; under argon.b Isolated yield.c Pd, 0.05 mol%. | ||
| 1 | 4-MeO | 99 |
| 2 | 4-CN | 98 |
| 3 | 4-tert-Butyl | 80 |
| 4 | 4-CHO | 99 |
| 5 | 4-MeCO | 99 |
| 6c | 2-MeO | 86 |
| 7c | 2-Me | 94 |
| 8c | 2-NO2 | 95 |
| 9c | 2-CN | 98 |
| 10c | 2,6-Di-Me | 93 |
Catalyst reusability and discussion
The reusability of the catalyst Pd@PNP was tested for the cross-coupling of 4-chlorotoluene with phenylboronic acid, and the yields with recycled catalyst Pd@PNP are presented in Fig. 2. The catalyst Pd@PNP can be reused 5 times at least, but slight deactivation of the Pd@PNP catalyst was observed. The deactivation may be due to the loss of the catalyst in the recycling processes and the slight aggregation of the Pd nanoparticles. And 4-phenyltoluene can be obtained in high yield also.To test the leakage of Pd@PNP catalyst, the content of Pd was measured by ICP-AES in the 5th reaction run solution, and no Pd was detected in the solution (below detection limit, <7 ppb). Moreover, no P (from the P ligand) was detected as having leaked into the solution. The solution was not active for the cross-coupling of 4-chlorotoluene with phenylboronic acid after removal of the catalyst Pd@PNP, which indicated that the catalyst Pd@PNP is a heterogeneous catalyst for Suzuki–Miyaura reaction. There was no contamination (ligand or Pd) found from the catalyst system, which is important for pharmaceutical chemistry.
In a one-pot process, Pd with tris(4-chlorophenyl)phosphine catalysed the coupling of tris(4-bromophenyl)amine with benzene-1,4-diboronic acid to form the polymer, and the tris(4-chlorophenyl)phosphine can also participate in the coupling reaction with benzene-1,4-diboronic acid. And then the triphenylphosphine (from tris(4-chlorophenyl)phosphine) was anchored into the polymer. Furthermore, active Pd nanoparticles were trapped and immobilized into the polymer. Evidently, active Pd nanoparticles were trapped and immobilized into the polymer preferentially, as the polymerization was catalyzed by the active Pd nanoparticles. Thus, highly active Pd@PNP catalyst can be obtained in the one-pot process.
Conclusions
In summary, we demonstrated a highly active and reusable Pd catalyst Pd@PNP for Suzuki–Miyaura reaction of aryl chlorides and bromides with aryl boronic acids, and the corresponding biphenyl compounds were obtained in good to excellent yields. The in situ immobilized triphenylphosphine in the polymer support enhanced the activity and the stability of the catalyst Pd@PNP, and the catalyst Pd@PNP can be reused several times with good activity. Moreover, the Pd@PNP catalyzed Suzuki–Miyaura reaction was applicable widely, and various aryl chlorides and bromides can be coupled with aryl boronic acids in high yields. Functional groups, such as methoxyl, nitrile, tert-butyl, nitro, acyl and formyl groups, were well tolerated under the reaction conditions, and the corresponding products were obtained in high yields.Acknowledgements
This work was supported by the fund from the State Key Laboratory of Materials-Oriented Chemical Engineering (grant ZK201402), the National High Technology Research and Development Program of China (No. 2012AA0 3A606) and the Project of Priority Academic Program Development of Jiangsu Higher Education Institutions.Notes and references
- H. Juteau, Y. Gareau, M. Labelle, C. F. Sturino, N. Sawyer, N. Tremblay, S. Lamontage, M. C. Carriere, D. Denis and K. M. Metters, Bioorg. Med. Chem., 2001, 9, 1977–1984 CrossRef CAS PubMed.
- (a) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS; (b) C. C. C. J. Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062–5085 CrossRef PubMed; (c) D. S. Surry and S. L. Buchwald, Chem. Sci., 2011, 2, 27–50 RSC.
- (a) A. Zapf, A. Ehrentraut and M. Beller, Angew. Chem., Int. Ed., 2000, 39, 4153–4155 CrossRef CAS; (b) A. F. Littke and G. C. Fu, Angew. Chem., Int. Ed., 2002, 41, 4176–4211 CrossRef CAS; (c) A. Thakur, K. Zhang and J. Louie, Chem. Commun., 2012, 48, 203–205 RSC; (d) C. Liu, X. Li, Z. Gao, X. Wang and Z. Jin, Tetrahedron, 2015, 71, 3954–3959 CrossRef CAS.
- (a) X. Wu, P. Anbarasan, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2010, 49, 9047–9050 CrossRef CAS PubMed; (b) F. Saleem, G. K. Rao, A. Kumar, S. Kumar and M. P. Singh, RSC Adv., 2014, 4, 56102–56111 RSC.
- (a) R. Chinchilla and C. Najera, Chem. Rev., 2007, 107, 874–922 CrossRef CAS PubMed; (b) B. H. Lipshutz, D. W. Chung and B. Rich, Org. Lett., 2008, 17, 3793–3796 CrossRef PubMed; (c) N. Liu, C. Liu, Q. Xu and Z. Lin, Eur. J. Org. Chem., 2011, 4422–4428 CrossRef CAS; (d) Y. Yong, J. F. Y. Lim, X. Y. Chew, E. G. Robins, C. W. Johannes, Y. H. Lim and H. Jong, Catal. Sci. Technol., 2015, 5, 3501–3506 RSC; (e) C. Dai and G. C. Fu, J. Am. Chem. Soc., 2001, 123, 2719–2724 CrossRef CAS PubMed; (f) C. Han and S. L. Buchwald, J. Am. Chem. Soc., 2009, 131, 7532–7533 CrossRef CAS PubMed; (g) H. Li, C. C. C. Johansson Seechurn and T. J. Colacot, ACS Catal., 2012, 2, 1147–1164 CrossRef CAS.
- (a) T. Tagata and M. Nishida, J. Org. Chem., 2003, 68, 9412–9415 CrossRef CAS PubMed; (b) Y. Kitamura, S. Sako, T. Udzu, A. Tsutsui, T. Maegawa, Y. Monguchi and H. Sajiki, Chem. Commun., 2007, 5069–5071 RSC; (c) S. Liu, H. Li, M. Shi, H. Jiang, X. Hu, W. Li, L. Fu and H. Chen, Macromolecules, 2012, 45, 9004–9009 CrossRef CAS.
- K. E. Balsane, R. S. Shelkar and J. M. Nagarkar, Catal. Lett., 2015, 145, 1817–1824 CrossRef CAS.
- (a) M. L. Kantam, S. Roy, M. Roy, B. Sreedhar and B. M. Choudary, Adv. Synth. Catal., 2005, 347, 2002–2008 CrossRef CAS; (b) S. Zhou, M. Johnson and J. G. C. Veinot, Chem. Commun., 2010, 46, 2411–2413 RSC; (c) B. Sreedhar, D. Yada and P. S. Reddy, Adv. Synth. Catal., 2011, 353, 2823–2836 CrossRef CAS; (d) H. Woo, K. Lee and K. H. Park, ChemCatChem, 2014, 6, 1635–1640 CrossRef CAS; (e) R. S. Shelkar, S. H. Gund and J. M. Nagarkar, RSC Adv., 2014, 4, 53387–53396 RSC.
- (a) T. V. Magdesieva, O. M. Nikitin, O. A. Levitsky, V. A. Zinovyeva, I. Bezverkhyy, E. V. Zolotukhina and M. A. Vorotyntsev, J. Mol. Catal. A: Chem., 2012, 353, 50–57 CrossRef; (b) Y. Zhou, C. Li, M. Lin, Y. Ding and Z. Zhan, Adv. Synth. Catal., 2015, 357, 2503–2508 CrossRef CAS; (c) Z. Ye, L. Xu, Z. Dong and P. Xiang, Chem. Commun., 2013, 49, 6235–6255 RSC; (d) D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852–7871 CrossRef CAS PubMed; (e) C. Ornelas, A. K. Diallo, J. Ruiz and D. Astruc, Adv. Synth. Catal., 2009, 351, 2147–2154 CrossRef CAS; (f) S. Ogasawara and S. Kato, J. Am. Chem. Soc., 2010, 132, 4608–4613 CrossRef CAS PubMed; (g) A. Modak, J. Mondal, M. Sasidharan and A. Bhaumik, Green Chem., 2011, 13, 1317–1331 RSC; (h) C. Deraedt, L. Salmon, J. Ruiz and D. Astruc, Adv. Synth. Catal., 2013, 355, 2992–3001 CrossRef CAS.
- Y. Li, F. Mao, T. Chen, Z. Zhou, Y. Wang and J. Huang, Adv. Synth. Catal., 2015, 357, 2827–2832 CrossRef CAS.
- Y. Yu, T. Hu, X. Chen, K. Xu, J. Zhang and J. Huang, Chem. Commun., 2011, 47, 3592–3594 RSC.
- (a) T. Hu, T. Schulz, C. Torborg, X. Chen, J. Wang, M. Beller and J. Huang, Chem. Commun., 2009, 7330–7332 RSC; (b) T. Hu, X. Chen, J. Wang and J. Huang, ChemCatChem, 2011, 3, 661–665 CrossRef CAS; (c) J. Zhang, X. Chen, T. Hu, Y. Zhang, K. Xu and Y. Yu, Catal. Lett., 2010, 139, 56–60 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra25925h |
| This journal is © The Royal Society of Chemistry 2016 |