Synthesis of bis(propargyl) aromatic esters and ethers: a potential replacement for isocyanate based curators†
Abstract
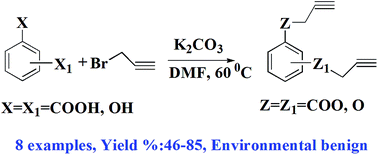
This study reports the synthesis and characterization of a novel class of non-isocyanate curing agents based on bis-propargyl aromatic esters 2a–e and ethers 4a–c. A total of eight non-isocyanate curators were prepared from the reaction of respective dicarboxy or dihydroxybenzene with propargyl bromide in the presence of potassium carbonate with good yields. The structure and purity of the synthesized compounds and the corresponding intermediates were confirmed by spectral (IR and NMR), thermal (DSC) and chromatographic techniques (HPLC & GC-MS). Furthermore, kinetics of the curing reaction between glycidyl azide polymer (GAP) and the synthesized alkynes (4b, 4c) were studied using time-resolved FT-IR spectroscopy as a function of time at 303, 323 and 333 K. It was found that the curing reaction was faster when the temperature was increased. Kinetic parameters of the curing reaction, such as the reaction order and activation energy, were calculated for the GAP–4a and GAP–4c systems. All the curing reactions followed first order kinetics and the corresponding activation energy of the curing reaction for the systems was found to be 15.56 and 13.22 kcal mol−1. For comparison, curing studies were performed for GAP with a conventional curator Desmodur N-100. GAP cured with non-isocyanate curators offered good mechanical properties compared to GAP cured with isocyanate (N-100). The advantage of these new curing systems is that they do not require catalyst and there is no need for specific environmental conditions. Based on these studies, 1,4-bis(2-propynyloxy)benzene (4b) has the most potential as a non-isocyanate curator for azide polymeric binders.

 Please wait while we load your content...
Please wait while we load your content...