Anionic hexadeca-carboxylate tetrapyrazinoporphyrazine: synthesis and in vitro photodynamic studies of a water-soluble, non-aggregating photosensitizer†
Abstract
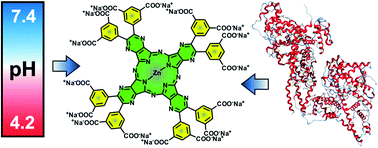
A sodium salt of zinc tetrapyrazinoporphyrazine bearing eight 3,5-dicarboxylatophenyl substituents (1) was synthesized. The presence of sixteen negative charges in a rigid arrangement on the periphery of the macrocycle inhibited its aggregation in water or buffers at pH > 5.8. Strong aggregation was, however, observed in buffers at pH < 4.8 due to the protonation of carboxylate functions. Fluorescence microscopy revealed that the compound localized to lysosomes and endosomes in cells. The compound's photodynamic activity on HeLa cancer cells (IC50 = 5.7 ± 1.1 μM) was found to be influenced by both pH and interactions with serum proteins. This was demonstrated with a detailed in vitro study based on the inhibition of vacuolar H+-ATPase using bafilomycin A1, which increased the intracellular fluorescence of 1. Compound 1 also formed interactions with serum proteins that partially quenched its excited states; however, they also protected the compound from self-aggregation at low pH.
- This article is part of the themed collection: Editors' Collection: Phthalocyanines

 Please wait while we load your content...
Please wait while we load your content...