High performance solution-processed infrared photodetector based on PbSe quantum dots doped with low carrier mobility polymer poly(N-vinylcarbazole)
Abstract
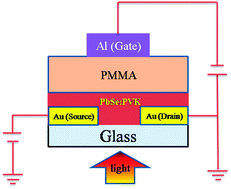
Colloidal quantum dots (CQDs) are promising materials for flexible electronics, light sensing and energy conversion. In particular, as a narrow bandgap semiconductor, lead selenide (PbSe) CQDs have attracted considerable interest due to their potential applications in infrared (IR) optoelectronics such as IR light-emitting diodes (LEDs), photodetectors and solar cells. Solution-processed photodetectors are more attractive owing to their flexible, large-scale and low-cost fabrication, and their performance depends greatly on the film quality and surface morphology. In this study, a high performance solution-processed infrared photodetector based on PbSe CQDs blended with low hole mobility polymer poly(N-vinylcarbazole) (PVK) is presented. In order to obtain a higher device performance, different volume ratios (K = VPVK/VPbSe) of PVK (20 mg ml−1 in chloroform) in PbSe CQDs (15 mg ml−1 in chlorobenzene) were investigated, and a maximum responsivity and specific detectivity of 2.93 A W−1 and 1.24 × 1012 jones, respectively, were obtained at VG = −20 V under 30 mW cm−2 980 nm laser illumination for field-effect transistor (FET)-based photodetector Au(S&D)/PbSe : PVK/PMMA/Al(G), in which PbSe : PVK nanocomposite with K = 1 : 2 acts as the active layer and poly (methyl methacrylate) (PMMA) as the dielectric layer. The reasons for the high device performance of PbSe : PVK nanocomposite as an active layer are discussed, in which PbSe nanoparticles were blended with low hole mobility polymer PVK but showed comparable detectivity as that blended with regioregular P3HT. Moreover, all these types of photodetectors are very stable for reverse fabrication using PMMA dielectric layer to shield the active layer from the environment and by inorganic ligand exchange treatment on the active layer.

 Please wait while we load your content...
Please wait while we load your content...