Potential hepatoprotective effects of fullerenol nanoparticles on alcohol-induced oxidative stress by ROS
Abstract
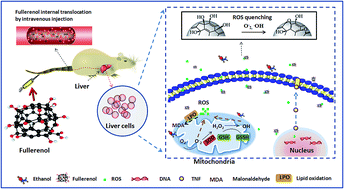
The free radical scavenging ability of fullerenols is their most exploited property in biomedical studies. The antioxidant properties and associated mechanisms of fullerenol have been previously investigated, but less research has been done to examine the hepatoprotective effects of fullerenol nanoparticles on alcohol-induced oxidative stress. This study examined the antioxidant effects in rat liver primary hepatocyte exposure to fullerenols dissolved in ethanol. In this study, liver primary hepatocytes of Wistar rats were divided into nine experimental groups that were exposed to ethanol (0.1, 1.0 and 10%), fullerenol dissolved in ethanol (0.1, 1.0 and 10%) and controls (PBS; PBS + fullerenol; Vitamin C (Vc) in 10% ethanol). The results demonstrated that fullerenol nanoparticles in 400 μmol L−1 exhibited excellent ROS scavenging abilities. This contributed to the anti-inflammatory effects involving reduced alcoholic oxidative damage and the regulated promotion of the tumor necrosis factor. The intracorporeal metabolism of rat-intake fullerenol was also evaluated by examining precision-cut rat slices of liver and kidney. All results demonstrated the potential hepatoprotective effects of fullerenol nanoparticles in the preventive treatment of alcoholic hepatopathy.

 Please wait while we load your content...
Please wait while we load your content...