Field emission properties of highly ordered low-aspect ratio carbon nanocup arrays†
Abstract
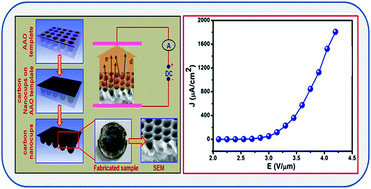
Herein, we design and develop a field emission device utilizing highly porous carbon nanocup (CNC) films. These three-dimensional (3D) low-aspect ratio CNC structures were fabricated by a combination of anodization and chemical vapor deposition techniques. The low turn-on fields of 2.30 V μm−1 were observed to draw an emission current density of 1 μA cm−2 and a maximum emission current density of ∼1.802 mA cm−2 drawn at an applied field of ∼4.20 V μm−1. The enhanced field emission behavior observed from the CNC films is attributed to an excellent field enhancement factor of 1645. The observed field emission properties of CNC arrays are attributed to a synergistic combination of high aspect ratio, nano-sized radius of curvature, highly organized distribution of the emitters over the whole area of specimen and lower screening effect of the CNC arrays. These observations shed light on the effect of the stacking carbon layers of CNC on their electronic properties and open up possibilities to integrate new morphologies of graphitic carbon in nanotechnology applications. Thus, the low turn on field, high emission current density and better emission current stability enable CNC based future field emission applications.

 Please wait while we load your content...
Please wait while we load your content...