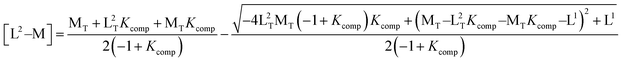A thiosemicarbazone based chemo and fluorogenic sensor for Zn2+ with CHEF and ESIPT behaviour: computational studies and cell imaging application†
Rahul Bhowmicka,
Rabiul Alama,
Tarun Mistria,
Kalyan Kumar Dasa,
Atul Katarkarb,
Keya Chaudhurib and
Mahammad Ali*a
aDepartment of Chemistry, Jadavpur University, Kolkata 700 032, India. E-mail: m_ali2062@yahoo.com
bMolecular & Human Genetics Division, CSIR-Indian Institute of Chemical Biology, 4 Raja S.C. Mallick Road, Kolkata-700032, India
First published on 20th January 2016
Abstract
We report herein the development of a novel, diformyl-p-cresol (DFC)–thiosemicarbazide (TS) based sensor (DFC–TS) that selectively and sensitively recognizes Zn2+ by both UV-Vis and fluorescence methods. The gradual addition of Zn2+ to a solution of the ligand developed a new absorption band at 430 nm, while the bands at 370 and 316 nm gradually decrease generating one well defined isosbestic point at 390 nm exhibiting ∼17 fold turn-on fluorescent enhancement (FE). When we plot absorbance (at 430 nm) vs. [Zn2+] there is a gradual increase in absorption with [Zn2+], becoming saturated at ∼1 equivalent of Zn2+ and then again it increases with the increase in [Zn2+] and ultimately becomes saturated at ∼2 equivalents of added Zn2+. This clearly demonstrates that the Zn2+ binding event to the ligand occurs in two steps, one at a time. Non-linear least-squares computer-fitting of these data gives the parameters: K′f1 = (9.70 ± 5.51) × 105 M−1, n = (1.28 ± 0.05) for the first step and K′f2 = (1.11 ± 0.65) × 105 M−1 and n = (1.01 ± 0.06) for the second step. So far, this study provides the opportunity where we have successfully, for the first time, determined the stepwise formation constants; though they have values of the same order of magnitude. The ground state geometries of DFC–TS, both enol and keto forms and [Zn(DFC–TS)(OAc)], [Zn(DFC–TS)(OAc)]−, and [Zn2(DFC–TS)(OAc)2] were optimized using the Gaussian-03 suit program and bond distances of all species are in reasonable agreement with the reported values.
Introduction
The creation of new tools and approaches for optical imaging in biology is an important goal, and investigations focused on these objectives will ultimately increase our understanding of many biological phenomena. Zn2+ is one of the most important metal ions in biology that is highly regulated under normal physiological conditions.1 The emerging importance of Zn2+ in neurological signaling and some proposed functions2 in biological systems have generated an urgent demand for the development of Zn2+-specific molecular probes,3 and many Zn2+ fluorescent sensors have been reported, exhibiting high selectivity and sensitivity over other biologically essential metal ions in specific ranges of concentration.4The mechanistic details of zinc homeostasis are not entirely clear,5 partly due to the difficulty in directly mapping the distribution and tracking the movement of the spectroscopically inert free zinc ions. Therefore, zinc ion-selective fluorescent probes have been the primary tools for the detection and quantification of biological free zinc. The past decade has witnessed the rapid progress in the development of zinc probes,6 which have enabled significant advances in the cell biology of zinc.7
Though the chemistry of thiosemicarbazones (Scheme 1) has been studied for a considerable period of time for their biological properties, the first report on their medicinal applications started to appear in the fifties as drugs against tuberculosis and leprosy.8 In the sixties their antiviral properties were discovered and a huge amount of research was carried out that eventually led to the commercialization of methisazone, Marboran® etc. to treat smallpox9 During this period the first antitumor activity results was published.10 Recently, triapine (3-aminopyridine-2-carboxaldehyde thiosemi-carbazone) has been developed as an anticancer drug for several cancer types.11
Understanding the excited-state proton/hydrogen-atom transfer Excited State Proton Transfer (ESPT) reaction is fundamental in chemistry and biology.12 Taylor reported the first case of excited state biprotonic transfer in 7-azaindole13 and Tahara14 proved that this proton transfer mechanism is a concerted process with the initial formation of a dimer in solution. Villani15 recently proved that the trans-formation of the Watson–Crick (WC) DNA base pairs to their tautomers is achieved via a concerted double-hydrogen transfer process beginning with a hydrogen atom of a purinic base. The ESIPT reaction in green fluorescent protein (GFP) has also attracted much interest,16 because it is a major tool in many areas of the life science. ESIPT molecules have attracted attention for various applications including chemical sensors,17 luminescent materials18 and functional polymers.19 A distinctive feature for the ESIPT molecules is that their fluorescence is well separated from their absorption maxima (>100 nm). This resulting unusually large Stokes shift is an obvious advantage over other normal fluorophores, such as fluorescein, rhodamine or coumarin, as they can avoid self-absorption and thus the inner filter effect in fluorescence analysis.
In the present article we are going to disclose a simple and easily synthesizable DFC–TS based turn-on colori- and fluorimetric dual sensor for Zn2+.The promise of this new ligand as a quantitative probe for Zn2+ is demonstrated by its use in the estimation of affinities towards Zn2+ through absorption and fluorescence changes occurring mainly due to ESIPT blocked Chelation Enhanced Fluorescence (CHEF) effect. Most striking feature of this study is that it enables us, for the first time, the direct determination of stepwise formation constants (K′f1 and K′f2); though they are of same order of magnitude and otherwise, very difficult to determine by any suitable analytical techniques. In addition, the ligand displays ESIPT behavior with large Stokes shift (>155 nm) that can avoid self-absorption and thus the inner filter effect in fluorescence analysis.
Experimental section
Materials and methods
The starting materials such as thiosemicarbazide (Sigma Aldrich), 2,6-diformyl-p-cresol (DFC, prepared in the laboratory) were used for the preparation of ligands DFC–TS. Zn(OAc)2·2H2O was used to prepare Zn2+ complexes. Solvent like DMSO, MeCN etc. (Merck, India) were of reagent grade.Physical measurements
Elemental analyses were carried out using a Perkin–Elmer 240 elemental analyzer. Infrared spectra (400–4000 cm−1) were recorded from KBr pellets on a Nickolet Magna IR 750 series-II FTIR spectrophotometers. 1H-NMR was recorded in DMSO-d6 on a Bruker 300 MHz NMR spectrometer using tetramethylsilane (δ = 0) as an internal standard. UV-Vis spectra were recorded on an Agilent diode-array spectrophotometer (Model, Agilent 8453), steady-state fluorescence were recorded on a Shimadzu spectrofluorimeter (Model RF-5301.) and PTI spectrofluorimeter (Model QM-40.), ESI-MS+ (m/z) of the ligand and Zn(II)-complex were recorded on a Waters' HRMS spectrometer (Model: XEVOG2 QTOF).Preparation of DFC–TS
2,6-Diformyl-p-cresol (DFC) was prepared by following a literature procedure.20 2,6-Diformyl-p-cresol (DFC) (1.64 g, 10 mmol) was dissolved in 25 ml EtOH under nitrogen atmosphere. To this solution was added thiosemicarbazide (1.82 g, 20 mmol) and stirred at room temperature for 5 h and then the reaction mixture was filtered to remove any undissolved materials and the filtrate was kept at room temperature. After 1 day a crystalline product was deposited. (Yield, 85.57%). 1H-NMR (in DMSO-d6) (δ, ppm): 2.24 (s, 3H, methyl), 2.48 (2-H, NH), 7.6285 (s, 2H, azomethine), 8.31 (s, 2-H, aromatic), 8.06 and 8.16 (s, 4H, NH2), 11.45 (s, 1-H, OH) (please see Fig. S1†). ESI-MS+ (m/z): 311.1094 (DFC–TS + H+), 316.0230 (DFC–TS + Li+), 333.0256 (DFC–TS + Na+). [Fig. S7†].Synthesis of [Zn(DFC–TS)(OAc)] and [Zn2(DFC–TS)(OAc)2]
To a solution of DFC–TS (0.62 g, 2 mmol, dissolved in few drops of DMSO) in 20 ml MeCN was added Zn(OAc)2·2H2O (0.498 g, 2 mmol in 10 ml MeCN solution) dropwise with continuous stirring. After 1 hour the solution was filtered and the filtrate was kept aside undisturbed. After one day crystalline [Zn(DFC–TS)(OAc)] was precipitated out. It was collected by filtration, washed several times with MeCN and dried in air. [Zn2(DFC–TS)(OAc)2] was prepared in the same way as that of 1, only 2 equivalents of Zn(OAc)2·2H2O was used here. Several trials to grow single crystals were failed. 1H-NMR (in DMSO-d6) (δ, ppm): 2.19 (s, 3H, methyl), 2.48 (2-H, NH), 6.93 (s, 2H, azomethine), 8.07 (s, 2-H, aromatic), 6.24 and 6.42 (s, 4H, NH2) for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Zn2+ complex and 2.19 (s, 3H, methyl), 2.52 (2-H, NH), 7.31 (s, 2H, azomethine), 8.31 (s, 2-H, aromatic), 6.43 (s, 4H, NH2) for 1
1 Zn2+ complex and 2.19 (s, 3H, methyl), 2.52 (2-H, NH), 7.31 (s, 2H, azomethine), 8.31 (s, 2-H, aromatic), 6.43 (s, 4H, NH2) for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Zn2+ complex, (please see Fig. S2 and S3†). ESI-MS+ (m/z): 372.9884, (DFC–TS + Zn2+) (1
2 Zn2+ complex, (please see Fig. S2 and S3†). ESI-MS+ (m/z): 372.9884, (DFC–TS + Zn2+) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 514.9107 ([Zn2(DFC–TS)(OAc)(H2O)]+) (please see Fig. S8 and S9†).
1), 514.9107 ([Zn2(DFC–TS)(OAc)(H2O)]+) (please see Fig. S8 and S9†).
Solution preparation for UV-Vis and fluorescence studies
For both UV-Vis and fluorescence titrations, a stock solutions of 1.0 × 10−3 M of the probe DFC–TS was prepared by dissolving the DFC–TS in 9 ml DMSO and finally the volume was made upto 10 ml by DMSO. Similarly, another 1.0 × 10−3 M stock solution of Zn2+ was prepared in de-ionised H2O. A solution of 10 mM HEPES buffer (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMSO
1 DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) was prepared and pH was adjusted to 7.2 by using HCl and NaOH. 2.5 ml of this buffer solution was pipetted out into a cuvette to which 20 μM of the probe was added and Zn2+ ion was added incrementally starting from 0 to 46 μM in a regular interval of volume and UV-Vis and fluorescence spectra were recorded for each solution. Path lengths of the cells used for absorption and emission studies were 1 cm. Fluorescence measurements were performed using 2 nm × 2 nm slit width.
H2O) was prepared and pH was adjusted to 7.2 by using HCl and NaOH. 2.5 ml of this buffer solution was pipetted out into a cuvette to which 20 μM of the probe was added and Zn2+ ion was added incrementally starting from 0 to 46 μM in a regular interval of volume and UV-Vis and fluorescence spectra were recorded for each solution. Path lengths of the cells used for absorption and emission studies were 1 cm. Fluorescence measurements were performed using 2 nm × 2 nm slit width.
For competition assays 1.0 × 10−3 M Na2H2EDTA solution was prepared in water. 2.5 ml of the buffered solution was pipetted out into a cuvette to which 20 μM of the DFC–TS was added and Zn2+ ion was added in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mole ratio separately and then Na2H2EDTA solution was added incrementally starting from 0 to 44 μM in a regular interval of volume and UV-Vis and fluorescence spectra were recorded for each solution.
2 mole ratio separately and then Na2H2EDTA solution was added incrementally starting from 0 to 44 μM in a regular interval of volume and UV-Vis and fluorescence spectra were recorded for each solution.
| Kcomp = [M–L2][L1]/[M–L1][L2]; Kcomp = KL2/KL1 |
Emission spectra were recorded by excitation at 430 nm. Both excitation and emission slit widths were 2 nm. The equilibrium competition constant (Kfi-comp) (i = 1 and 2 for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 L
2 L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M mole ratios respectively) were calculated based on the titration curve.
M mole ratios respectively) were calculated based on the titration curve.
Job's study
This method is based on the measurement of a series of solutions in which molar concentrations of two reactants vary but their sum remains constant. The absorbance of each solution is measured at a suitable wavelength and plotted against the mole fraction of one of the reactants. A maximum in absorbance occurs at the mole ratio corresponding to the combining ratio of the reactants.Computational details
Ground state electronic structure calculations of DFC–TS (both enol and keto forms) and complexes (both mono and dinuclear species) have been carried out using Gaussian-03 suit program.22 Becke's hybrid function23 with the Lee–Yang–Parr (LYP) correlation function24 were used along with 6-31+G(d,p) basis set for H, C, N, O atoms and LANL2DZ effective core potentials and basis set for the Zn atom. The global minima of all these species were confirmed by the positive vibrational frequencies. Time dependent density functional theory (TDDFT)25,26 with B3LYP density functional associated with the conductor-like polarizable continuum model (CPCM)27 were applied to study the low-lying excited states of the ligand and the complexes in DMSO using the optimized geometry of the ground (S0) state. The vertical excitation energies of the lowest 20 singlet states are also computed here (Table S4†).Cell culture
HepG2 cell lines, human hepatocellular liver carcinoma cells, were procured from National Center for Cell Science, Pune, India, and used throughout the study. Cells were cultured in DMEM (Gibco BRL) supplemented with 10% FBS (Gibco BRL), and a 1% antibiotic mixture containing Penicillin, streptomycin and gentamicin (Gibco BRL) at 37 °C in a humidified incubator with 5% CO2 and cells were grown to 60–80% confluence, harvested with 0.025% trypsin (Gibco BRL) and 0.52 mM EDTA (Gibco BRL) in phosphate-buffered saline (PBS), plated at the desired cell concentration and allowed to re-equilibrate for 24 h before any treatment.Cell cytotoxicity assay
To test the cytotoxicity of DFC–TS assay was performed as per the procedure described earlier.28 After treatment with DFC–TS at different doses of 1, 10, 20, 50 and 100 μM, respectively, for 12 h, 10 μl of MTT solution (10 mg ml−1 PBS) was added to each well of a 96-well culture plate and again incubated continuously at 37 °C for a period of 3 h. All media were removed from wells and 100 μl acidic isopropyl alcohol was added into each well. The intracellular formazan crystals (blue-violet) formed were solubilized with 0.04 N acidic isopropyl alcohol and absorbance of the solution was measured at 595 nm with a microplate reader (Model: THERMO MULTI SCAN EX). The cell viability was expressed as the optical density ratio of the treatment to control. Values were expressed as mean ± standard errors of three independent experiments. The cell cytotoxicity was calculated as % cell cytotoxicity = 100% − % cell viability.Cell imaging study
HepG2 Cells were incubated with 10 μM DFC–TS [the stock solution (1 mM) was prepared by dissolving DFC–TS to the mixed solvent (DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water = 1
water = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (v/v))] in the culture medium, allowed to incubate for 30 min at 37 °C. After incubation, cells were washed twice with phosphate-buffered saline (PBS). Bright field and fluorescence images of HepG2 cells were taken by a fluorescence microscope (Leica DM3000, Germany) with an objective lens of 40× magnification. Fluorescence images of HepG2 cells were taken separately from another set of experiment where cells incubated with 10 μM DFC–TS + 10 μM Zn2+ for 30 min. Similarly, in another set of experiment, cells were incubated with 10 μM DFC–TS + 10 μM Zn2+ for 30 min followed by addition of 100 μM EDTA for another 30 min and fluorescence images were taken. HepG2 cells showed almost complete quenching of fluorescence due to removal of Zn2+ from the DFC–TS–Zn2+ complex.
9 (v/v))] in the culture medium, allowed to incubate for 30 min at 37 °C. After incubation, cells were washed twice with phosphate-buffered saline (PBS). Bright field and fluorescence images of HepG2 cells were taken by a fluorescence microscope (Leica DM3000, Germany) with an objective lens of 40× magnification. Fluorescence images of HepG2 cells were taken separately from another set of experiment where cells incubated with 10 μM DFC–TS + 10 μM Zn2+ for 30 min. Similarly, in another set of experiment, cells were incubated with 10 μM DFC–TS + 10 μM Zn2+ for 30 min followed by addition of 100 μM EDTA for another 30 min and fluorescence images were taken. HepG2 cells showed almost complete quenching of fluorescence due to removal of Zn2+ from the DFC–TS–Zn2+ complex.
Results and discussion
2,6-Diformyl-p-cresol (DFC, 1)−thiosemicarbazide (TS, 2) based (DFC–TS) conjugate was synthesized by a simple Schiff base condensation in EtOH as outlined in Scheme 1. This ligand has potentially penta-coordinating N2S2O donor atoms that can bind to one or two metal ions leading to the formation of mono- and dinuclear complexes. The DFC–TS ligand and its Zn2+ complexes have been isolated and characterized by various physicochemical (NMR (Fig. S1–S5†), IR (Fig. S6†) and ESI-MS+ (Fig. S7–S9†) (m/z)) methods.UV-Vis absorption studies
Intramolecular hydrogen bonded organic chromophores often exhibit the photophysical phenomenon like (ESIPT). The process of ESIPT is an intramolecular version of the chemistry of photo acids and photo bases whereby a molecule contains both acidic and basic groups in appropriate proximity and the acidic proton can migrate to the basic site upon excitation. Most ESIPT processes are aided by either six-or five-membered strong intramolecular hydrogen bonds in the ground state and usually require very little movement of nuclei. When solvent is not required to mediate such proton transfer process, it is described as intrinsic ESIPT and is barrier less in non-polar solvents occurring in the sub-picoseconds time region.29 The first reported ESIPT reaction is observed in methyl salicylate.30The electronic absorption spectral properties of DFC–TS were investigated in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMSO–H2O at pH 7.2 HEPES buffer. The absorption spectrum of DFC–TS presents two important transitions with intense absorption bands at 316 and 370 nm. As Scheme 2 describes, there is an equilibrium between the two configurations, enol and keto tautomers of DFC–TS due to ESIPT. The band at 370 nm is assigned to the transition to quinoid (keto) form. On gradual addition of Zn2+, the absorption bands of DFC–TS at 370 and 316 nm decrease gradually; however, a new peak appears at 430 nm thereby generating one well defined isosbestic point at 390 nm indicating a clean transformation of free ligand to its metal bound state (Fig. 1, S10 and S11†). This clearly indicates the chelation of Zn2+ by the multidentate ligand moiety giving [[Zn(DFC–TS)]+ and [Zn2(DFC–TS)]+]. The spectral change (decrease in absorbance at 370 nm and increase at 430 nm) clearly indicate that the addition of Zn2+ ion induces the decrease in concentration of free ligand both in keto and enol forms. Here the nitrogen atom of the azomethine group takes part in the coordination with Zn2+ and the formation of complexes Zn–DFC–TS and Zn2–DFC–TS inhibit the ESIPT (Scheme 2).
1 DMSO–H2O at pH 7.2 HEPES buffer. The absorption spectrum of DFC–TS presents two important transitions with intense absorption bands at 316 and 370 nm. As Scheme 2 describes, there is an equilibrium between the two configurations, enol and keto tautomers of DFC–TS due to ESIPT. The band at 370 nm is assigned to the transition to quinoid (keto) form. On gradual addition of Zn2+, the absorption bands of DFC–TS at 370 and 316 nm decrease gradually; however, a new peak appears at 430 nm thereby generating one well defined isosbestic point at 390 nm indicating a clean transformation of free ligand to its metal bound state (Fig. 1, S10 and S11†). This clearly indicates the chelation of Zn2+ by the multidentate ligand moiety giving [[Zn(DFC–TS)]+ and [Zn2(DFC–TS)]+]. The spectral change (decrease in absorbance at 370 nm and increase at 430 nm) clearly indicate that the addition of Zn2+ ion induces the decrease in concentration of free ligand both in keto and enol forms. Here the nitrogen atom of the azomethine group takes part in the coordination with Zn2+ and the formation of complexes Zn–DFC–TS and Zn2–DFC–TS inhibit the ESIPT (Scheme 2).
The cation binding affinities of sensor toward Zn2+ was investigated by UV-Vis absorption studies and when a change in absorbance at 430 nm was plotted against [Zn2+], there is a gradual increase in absorbance with [Zn2+], gets saturated at ∼1 equivalent of Zn2+ and then it again increases with the increase in [Zn2+] and finally get saturated at ∼2 equivalent of added Zn2+ (inset (b), Fig. 1). This clearly demonstrates that the Zn2+ binding event to the ligand occurs in two steps, one at a time. Non-linear least-squares fit of the data to eqn (1)
| y = (a + b × c × xn)/(1 + c × xn),21 | (1) |
| Formation constant | Spectrophotometrically | Fluorometrically |
|---|---|---|
| K′f1 | (9.7 ± 5.51) × 105 M−1 | (7.17 ± 0.2) × 104 M−1 [(1.14 ± 0.47) × 105 M−1; λex = 430 nm] |
| K′f2 | (1.11 ± 0.65) × 105 M−1 | — |
| K′f1 (competition) | (2.28 ± 0.32) × 105 M−1 | (1.38 ± 0.29) × 105 M−1 |
| K′f2 (competition) | (1.64 ± 0.17) × 105 M−1 | (7.2 ± 2.3) × 104 M−1 |
We have also attempted to determine the formation constants by competition method by reacting the in situ generated complexes like [Zn(DFC–TS)] and [Zn2(DFC–TS)] with Na2H2EDTA and equilibrium competition constants (K′f1 and K′f2) were calculated from the plots of (Amax − A)/Amax as a function of H2EDTA2− concentration by adopting displacement model. The evaluated apparent formation constants are: K′f1 = (2.28 ± 0.32) × 105 M−1 and K′f2 = (1.64 ± 0.17) × 105 M−1 and corresponding formation constants Kf1 and Kf2 were calculated from the relation Kfi = K′EDTA/K′fi where K′EDTA = KEDTA × α4 = 2.55 × 1013 and α4 = K1K2K3K4/([H+]4 + K1[H+]3 + K1K2[H+]2 + K1K2K3[H+] + K1K2K3K4) = 7.98 × 10−4 at pH 7.20 with K1 = 1.02 × 10−2, K2 = 2.14 × 10−3; K3 = 6.92 × 10−7 and K4 = 5.50 × 10−11 and KZn-EDTA = 3.2 × 1016.31 So the calculated values are Kfi = 1.55 × 108 and Kf2 = 1.11 × 108. The apparent formation constant values (K′f1 = (2.28 ± 0.32) × 105 M−1 and K′f2 = (1.64 ± 0.17) × 105 M−1) are found to be very close to the values obtained by non-linear fitting of absorption data as a function of [Zn2+] (Fig. S12†).
The composition of the complex was determined by Job's method and it was revealed that an overall composition is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with respect to the Zn2+ ion.
1 with respect to the Zn2+ ion.
We have also spectroscopically determined the acid dissociation constants of the free ligand (DFC–TS) and its Zn2+ complex with slightly excess over two equivalents of Zn2+ ions by monitoring the absorption of free ligand at 430 nm as a function of pH spanning between 2.0 and 12.0 using the eqn (2)
| A = (AAH [H+] + AA−Ka)/(Ka + [H+]),32,33 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMSO–water, v/v) compared to the free 4-methylphenol (19.1 and 10.2 in DMSO and pure water respectively).35
1 DMSO–water, v/v) compared to the free 4-methylphenol (19.1 and 10.2 in DMSO and pure water respectively).35
 | ||
Fig. 2 Determination of acid dissociation constant of (a) DFC–TS (free) and (b) DFC–TS in presence of 2.1 equivalents of Zn2+ in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMSO–water (v/v) at 25 °C. 1 DMSO–water (v/v) at 25 °C. | ||
The emission spectra of DFC–TS and its fluorescence titration with Zn2+ were recorded in DMSO–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.20, HEPES buffer) (Fig. 3) at λex = 390 nm. The binding between the free DFC–TS and Zn2+ leads to chelation enhanced fluorescence (CHEF) effect causing ∼17 fold fluorescence intensity enhancement due to increased structural rigidity of the formed complexes (Fig. 3).36 On excitation at 390 nm, the isosbestic point in absorption spectra, there is gradual increase in fluorescence intensity (FI) at λem = 500 nm, with the increase in [Zn2+] upto 1
1, v/v, pH 7.20, HEPES buffer) (Fig. 3) at λex = 390 nm. The binding between the free DFC–TS and Zn2+ leads to chelation enhanced fluorescence (CHEF) effect causing ∼17 fold fluorescence intensity enhancement due to increased structural rigidity of the formed complexes (Fig. 3).36 On excitation at 390 nm, the isosbestic point in absorption spectra, there is gradual increase in fluorescence intensity (FI) at λem = 500 nm, with the increase in [Zn2+] upto 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio, after that on increasing [Zn2+] there is a blue shift in λem from 500 to 490 nm (Fig. 3). This observation clearly indicates the stepwise uptake of Zn2+ ions in the ligand framework. The linear dependence of FI as function of [Zn2+] upto 1
1 mole ratio, after that on increasing [Zn2+] there is a blue shift in λem from 500 to 490 nm (Fig. 3). This observation clearly indicates the stepwise uptake of Zn2+ ions in the ligand framework. The linear dependence of FI as function of [Zn2+] upto 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio was analyzed with the help of eqn (1) in the form y = a + b × c × x, under the conditions 1 ≫ c × x and n = 1. It is interesting to note that linear least-squares analysis of fluorescence titration data gives K′f (7.17 ± 0.20) × 104 M−1. In addition, at λex = 430 nm there is a gradual blue shift of λem from 520 nm for pure ligand to 500 nm on complexation with Zn2+ and may be a consequence of slight involvement of Intramolecular Charge Transfer (ICT).37 It is interesting to note that non-linear least-squares analysis of fluorescence titration data gives K′f (1.14 ± 0.47) × 105 M−1 and n = (1.03 ± 0.04) and it is obvious that on gradual addition of Zn2+ fluorescence intensity at λem 500 nm increases and gets saturated at ∼DFC–TS
1 mole ratio was analyzed with the help of eqn (1) in the form y = a + b × c × x, under the conditions 1 ≫ c × x and n = 1. It is interesting to note that linear least-squares analysis of fluorescence titration data gives K′f (7.17 ± 0.20) × 104 M−1. In addition, at λex = 430 nm there is a gradual blue shift of λem from 520 nm for pure ligand to 500 nm on complexation with Zn2+ and may be a consequence of slight involvement of Intramolecular Charge Transfer (ICT).37 It is interesting to note that non-linear least-squares analysis of fluorescence titration data gives K′f (1.14 ± 0.47) × 105 M−1 and n = (1.03 ± 0.04) and it is obvious that on gradual addition of Zn2+ fluorescence intensity at λem 500 nm increases and gets saturated at ∼DFC–TS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn2+ = 1
Zn2+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 when exited at 430 nm (Fig. S13†). On further addition of Zn2+ there is no visible increase/decrease in fluorescence intensity.
1 when exited at 430 nm (Fig. S13†). On further addition of Zn2+ there is no visible increase/decrease in fluorescence intensity.
 | ||
Fig. 3 Fluorescence titration of DFC–TS (20 μM) with Zn2+ (0–48 μM) in DMSO–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.20, 10 mM HEPES buffer), λex = 390 nm, R2 = 0.99. 1, v/v, pH 7.20, 10 mM HEPES buffer), λex = 390 nm, R2 = 0.99. | ||
Again, when we added Na2H2EDTA to an in situ generated [Zn(DFC–TS)] (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 L
1 L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M) and [Zn2(DFC–TS)] (1
M) and [Zn2(DFC–TS)] (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 L
2 L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M) complexes the observations are same as in case of absorption titration i.e. there is a gradual decrease in absorbance with the increase in Na2H2EDTA concentration (Fig. 4 and S14†). However, here the phase separation (2
M) complexes the observations are same as in case of absorption titration i.e. there is a gradual decrease in absorbance with the increase in Na2H2EDTA concentration (Fig. 4 and S14†). However, here the phase separation (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes) is quite distinct thereby facilitating the determination of Kf1 and Kf2 unambiguously which were evaluated to be Kf1 = 1.03 × 108 and Kf2 = 0.92 × 108. These values are in good agreement to those obtained by absorption titration data.
1 complexes) is quite distinct thereby facilitating the determination of Kf1 and Kf2 unambiguously which were evaluated to be Kf1 = 1.03 × 108 and Kf2 = 0.92 × 108. These values are in good agreement to those obtained by absorption titration data.
 | ||
Fig. 4 (a) Plot of FI as a function of [Na2H2EDTA ] for the competitive displacement of Zn2+ from [Zn2(DFC–TS)] ensemble (20 μM with respect to DFC–TS) by Na2H2EDTA (0–44 μM) in DMSO-H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) at pH 7.2 (HEPES buffer) and (b) non-linear fitting of data using eqn (1). 1, v/v) at pH 7.2 (HEPES buffer) and (b) non-linear fitting of data using eqn (1). | ||
The stoichiometries of the Zn2+ complexes of DFC–TS as delineated by UV-VIS and fluorescence titrations were further confirmed by ESI-MS+ (m/z) mass spectrometry. The prominent ESI-MS+ peak of the pure ligand at 311.1094, 316.0230 and 333.0256 corresponds to [DFC–TS + H+], [DFC–TS + Li+] and, [DFC–TS + Na+], respectively. The mass spectrum of the isolated [Zn(DFC–TS)]+ complex at m/z = 372.9884 corresponds to [Zn(DFC–TS)]+ indicating a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation in the initial stage. However the presence of a very small peak for 1
1 complexation in the initial stage. However the presence of a very small peak for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex [Zn2(DFC–TS)(OAc)(H2O)]+ in solution is also apparent (Fig. S8†). When we prepared the solid product by reacting DFC–TS and Zn2+ in 1
2 complex [Zn2(DFC–TS)(OAc)(H2O)]+ in solution is also apparent (Fig. S8†). When we prepared the solid product by reacting DFC–TS and Zn2+ in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mole ratio the a prominent ESI-MS+ peak appears at 514.9107 (m/z) that indicating the presence of complex species [Zn2(DFC–TS)(OAc)(H2O)]+ in solution. However no peaks appear for 1
2 mole ratio the a prominent ESI-MS+ peak appears at 514.9107 (m/z) that indicating the presence of complex species [Zn2(DFC–TS)(OAc)(H2O)]+ in solution. However no peaks appear for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex.
1 complex.
The coordination modes were further supported by 1H-NMR studies. 1H-NMR peaks appear at 7.68, 8.31 and 11.45 ppm, correspond to azomethine proton (a), aromatic proton (b) and phenolic proton (c) and also peaks at 8.07 and 8.16 correspond to NH2 proton. The appearance of two separate peaks for NH2 proton may be due to the presence of two NH2 groups in two different chemical environments. The appearance of OH proton signal at >10.0 ppm signifies the presence of H-bonding. This peak intensity decreases significantly for [Zn(DFC–TS)]+ (1) and vanishes completely in case of dinuclear complex (2) indicating that phenolate group in DFC–TS participates in complexation with Zn2+ but in solution there is certain extent of free ligand in the case of complex 1. The 1H-NMR peak of azomethine proton (a) shifted to high field region (lower ppm, 6.93 for complex 1 and 7.31 for complex 2) reflecting the fact that the azomethine nitrogen participated in complexation. The aromatic protons remain almost invariant in the three species. Similar is the case for methyl proton. The coordination through sulphur atom to the Zn2+ is confirmed by IR spectra, where free ligand gives IR strectching frequency centered at 1110 cm−1 for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond (normal range 1050–1200 cm−1) (Fig. 5) which on complexation splits into two peaks appearing at 1110 and 1090 cm−1. The shifting to smaller frequency clearly points out the lowering in electron density around the C–S bond and corresponds to the existence of thiolate ion.
S bond (normal range 1050–1200 cm−1) (Fig. 5) which on complexation splits into two peaks appearing at 1110 and 1090 cm−1. The shifting to smaller frequency clearly points out the lowering in electron density around the C–S bond and corresponds to the existence of thiolate ion.
 | ||
| Fig. 5 IR spectra of DFC–TS ligand (shown in black), (Zn–DFC–TS) complex (shown in green), (Zn2–DFC–TS) complex (shown in red) in solid KBr-pellets. | ||
The detection of Zn2+ was not perturbed by biologically abundant Na+, K+, Ca2+, Mg2+, Al3+ etc. metal ions. Several transition metal ions, namely Cr3+, Mn2+, Fe2+, Fe3+, Co2+, Ni2+, Cu2+, and heavy metal ions like Cd2+, Pb2+, and Hg2+, also caused no interference (Fig. 6). The sensor was found to bind Zn2+ reversibly as tested by reacting with excess EDTA in intra-cellular conditions and also with EDTA in extra-cellular conditions. Moreover, no significant change in emission spectra was observed even on excess addition of the above mentioned interfering metal ions.
 | ||
| Fig. 6 Fluorescence and histogram plots to indicate the selectivity of DFC–TS toward Zn2+ over other biologically relevant metal ions, λex = 430 nm. | ||
For practical application, the appropriate pH conditions for successful operation of the sensor were evaluated. The probe DFC–TS fluoresces rather very weakly between pH 2 and 8 while Zn–DFC–TS complexes fluoresce extensively between pH 5 and 8 in 10 mM HEPES buffer, clearly indicating that this pH range is suitable for fluorescence studies for the complexation with Zn2+ (Fig. 7). The fluorescence of free ligand DFC–TS increases enormously beyond pH 10.0 and may be due to the acid dissociation of the thioamide proton.
 | ||
Fig. 7 pH depence of FI of the free ligand, DFC–TS and [Zn2(DFC–TS)]+ with DFC–TS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn = 1 Zn = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.15 in 10 mM HEPES buffer in 9 2.15 in 10 mM HEPES buffer in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMSO 1 DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v) solvent system, λex = 430 nm. H2O (v/v) solvent system, λex = 430 nm. | ||
The intracellular Zn2+ imaging behaviour of DFC–TS was studied on HepG2 cells with the aid of fluorescence microscopy which displayed intensive fluorescence when cells pre-incubated with DFC–TS were exposed to Zn2+ ion (10.0 μM), (Fig. 8) which, however, strongly suppressed when Na2H2EDTA (100 μM) was added (Fig. 8). Therefore, DFC–TS renders a confirmatory evidence of the intracellular monitoring of Zn2+ without interference by biologically abundant other metal ions. We have also determined quantum yields of the ligand, [Zn2(DFC–TS)] complex, which showed higher quantum yield for Zn2+complex (0.143) than the pure ligand (0.0074).
Geometry optimization and electronic structure
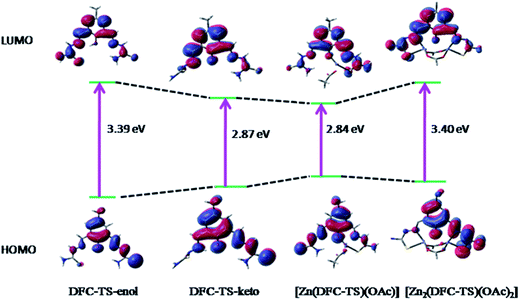
Geometries of DFC–TS and mono [Zn(DFC–TS)(OAc)] (1) and dinuclear [Zn2(DFC–TS)(OAc)2] (2) complexes were fully optimized using B3LYP functional as implemented in Gaussian 03.22 The nature of all the stationary points was confirmed by carrying out a normal mode analysis, where all vibrational frequencies were found to be positive. Some selected optimized geometrical parameters of DFC–TS for both keto and enol forms and complexes 1 and 2 are listed in Table S1 and S2 (ESI†) and geometry optimized structures are given in Fig. 9. The HOMO–LUMO gap for keto (2.87 eV) and enol (3.39 eV) decidedly indicates that the later is more stable than the former. The modelled geometry of complex 1 showed a penta-coordination around the metal centre in a distorted trigonal-bipyrimidal fashion. Here, phenoxo-O, azomethyne-N, keto-S and two oxygen atoms from acetate group participate in binding to Zn2+ giving a neutral species while other thiosemicabazone group rather remains dangling without participating in bonding to the metal center.We have also optimized the geometry of complex 1 as an anionic species where sulphide (S−) from C–SH (enolic form) coordinates to the metal center, other coordinating atoms remain unchanged. It is to be mentioned here that neutral species is more stable than the anionic species as indicated by the HOMO → LUMO energy gap (Fig. 10). The complex 2 is a dinuclear neutral species where one DFC–TS with N2S2O donor set of atoms and 4 oxygen atoms from two acetate groups involved in bonding. The phenoxo-O atom acts as a bridge between two metal centers along with two bridging acetate groups to hold the Zn2+ atoms firmly resulting distorted TBP geometries around them. The idea of such geometry as a dinuclear complex comes from our previous structural studies with analogous ligand. The bond distances and bond angles (Table S1 and S2 in ESI†) around the metal center(s) are very similar to those found in analogous complexes. In 1 and 2 all the calculated Zn–N/Zn–O distances fall in the range 2.054–2.319 Å and comparable to the reported values in the analogous complexes.36,38
Time dependent density functional theory (TDDFT)23–25 with B3LYP density functional was applied to study the low-lying excited states of the complex in DMSO using the optimized geometry of the ground (S0) state. The vertical excitation energies of the lowest 20 singlet states are also computed here. The UV-Vis spectra computed from TDDFT calculations in DMSO show two important peaks in the range 250–600 nm (see Fig. S6 and S7†) for DFC–TS. For complex 1, the band around 370 nm is dominated by the HOMO → LUMO excitations, while the band around ∼320 nm is mainly due to HOMO−1 → LUMO, HOMO−1 → LUMO+1 and HOMO → LUMO+1 transition. The corresponding experimental absorption bands appear at 370 nm and 317 nm respectively. In case of [Zn(DFC–TS)(OAc)] the absorption band around ∼420 nm is dominated by the HOMO → LUMO excitations, while the band around ∼340 nm it is mainly due to HOMO−1 → LUMO, and HOMO → LUMO+1 transitions. The absorption band at ∼300 nm arises mainly due to HOMO−3 → LUMO transition. The corresponding experimental absorption bands appear at 430 nm and 300 nm. Similarly, in case of [Zn2(DFC–TS)(OAc)2] the absorption bands at∼ 420 nm is dominated by the HOMO → LUMO excitations, while the band around ∼340 nm is mainly due toHOMO−1 → LUMO, and HOMO → LUMO+1 transitions. The TDDFT calculation on the mono- and dinuclear complexes reveal that there is no basic differences in the pattern of absorptions spectra of these species which is also evidenced from the experimental spectra where the λmax (absorption) remains almost unaltered for these two species but absorption continues to increase on adding Zn2+ beyond 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ration, until it gets saturated at 2
1 mole ration, until it gets saturated at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio with respect to Zn2+. The details of the vertical excitation energies, oscillator strengths, and nature of excitations are shown in Table S3 (ESI†). HOMO–LUMO diagrams of all the optimized species along with the energy gaps are depicted in Fig. 10.
1 mole ratio with respect to Zn2+. The details of the vertical excitation energies, oscillator strengths, and nature of excitations are shown in Table S3 (ESI†). HOMO–LUMO diagrams of all the optimized species along with the energy gaps are depicted in Fig. 10.
A list of 2,6-diformyl-p-cresol based Schiff base ligands36,39–43 that showed Zn2+ sensing is given in Scheme 3.
A quick inspection of these studies reveal that all these are turn on Zn2+ sensor with moderate LOD and find application towards intracellular Zn2+ monitoring.36,39–43
Conclusion
In summary, we have been successful to design and synthesize a DFC–TS based penta-coordinating N2S2O donor ligand that selectively recognizes Zn2+ colorimetrically as well as fluorimetrically with 17 fold fluorescence enhancement. The fluorescence enhancement occurs through ESIPT blocked chelation enhanced fluorescence effect (CHEF). Though, previously it was found that dithiosemicabazone based probes have very poor aqueous solubility and form complexes with Zn2+ very slowly44 precluding the direct determination of formation constants, the present Zn2+ sensor provides opportunity for the first time, to the direct determination of step-wise formation constants of similar order of magnitude by UV-Vis titration. This is possibly due to the reorganization of the ligand environment of the mononuclear complex to accommodate the second Zn2+ ion to form the dinuclear species. This probe has cell penetrating ability thereby providing opportunity for in vitro/in vivo cell imaging of Zn2+ ion without much worry about cytotoxicity.Acknowledgements
Financial support from DST (Ref. SR/S1/IC-35/2006) and CSIR (ref. 02(2490)/11/EMR-II) New Delhi, are gratefully acknowledged.Notes and references
- B. L. Vallee and K. H. Falchuk, Physiol. Rev., 1993, 73, 79–118 CAS.
- C. J. Frederickson, J. Y. Koh and A. I. Bush, Nat. Rev. Neurosci., 2005, 6, 449–462 CrossRef CAS PubMed.
- (a) S. C. Burdette and S. J. Lippard, Coord. Chem. Rev., 2001, 333, 216–217 Search PubMed; (b) S. Sreejith, K. P. Divya and A. Ajayaghosh, Chem. Commun., 2008, 2903–2905 RSC; (c) K. P. Divya, S. Sreejith, B. Balakrishna, P. Jayamurthy, P. Aneesa and A. Ajayaghosh, Chem. Commun., 2010, 46, 6069–6071 RSC; (d) S. Sreejith, K. P. Divya, P. Jayamurthy, J. Mathew, V. N. Anupama, D. S. Philips, P. Aneesa and A. Ajayaghosh, Photochem. Photobiol. Sci., 2012, 11, 1715–1723 RSC; (e) K. P. Divya, S. Sreejith, P. Ashokkumar, K. Yuzhan, Q. Peng, S. K. Maji, Y. Tong, H. Yu, Y. Zhao, P. Ramamurthy and A. Ajayaghosh, Chem. Sci., 2014, 5, 3469–3474 RSC; (f) A. Ajayaghosh, P. Carol and S. Sreejith, J. Am. Chem. Soc., 2005, 127, 14962–14963 CrossRef CAS PubMed; (g) E. L. Que, R. Bleher, F. E. Duncan, B. Y. Kong, S. C. Gleber, S. Vogt, S. Chen, S. A. Garwin, A. R. Bayer, V. P. Dravid, T. K. Woodruff and T. V. O'Halloran, Nat. Chem., 2015, 7, 130–139 CrossRef CAS PubMed.
- E. M. Nolan, J. Jaworski, K. I. Okamoto, Y. Hayashi, M. Sheng and S. J. Lippard, J. Am. Chem. Soc., 2005, 127, 16812–16823 CrossRef CAS PubMed.
- D. H. Nies, Science, 2007, 317, 1695–1696 CrossRef CAS PubMed.
- (a) K. Kikuchi, H. Komatsu and T. Nagano, Curr. Opin. Chem. Biol., 2004, 8, 182–191 CrossRef CAS PubMed; (b) S. Goswami, A. K. Das, K. Aich, A. Manna, S. Maity, K. Khanrab and N. Bhattacharyya, Analyst, 2013, 138, 4593–4598 RSC; (c) S. Goswami, S. Paul and A. Manna, RSC Adv., 2013, 3, 25079–25085 RSC; (d) S. Goswami, A. Manna, S. Paul, A. K. Maity, P. Saha, C. K. Quah and H. K. Fun, RSC Adv., 2014, 4, 34572–34576 RSC.
- C. J. Chang and S. J. Lippard, Life Sci., 2006, 1, 321–370 CAS.
- E. M. Bavin, R. J. W. Rees, J. M. Robson, M. Seiler, D. E. Seymour and D. J. Suddaby, J. Pharm. Pharmacol., 1950, 2, 764–772 CrossRef CAS PubMed.
- G. A. Kune, Br. Med. J., 1964, 2, 621–622 CAS.
- A. C. Sartorelli and B. A. Booth, Cancer Res., 1967, 27, 1614–1619 CAS.
- C. M. Nutting, C. M. L. vanHerpen, A. B. Miah, S. A. Bhide, J. P. Machiels, J. Buter, C. Kelly, D. de Raucourt and K. J. Harrington, Ann. Oncol., 2009, 20, 1275–1279 CrossRef CAS.
- J. Waluk, Acc. Chem. Res., 2003, 36, 832–838 CrossRef CAS PubMed.
- C. A. Taylor, M. A. El-Bayoumi and M. Kasha, Proc. Natl. Acad. Sci. U. S. A., 1969, 63, 253–260 CrossRef CAS.
- S. Takeuchi and T. Tahara, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 5285–5290 CrossRef CAS PubMed.
- G. Villani, J. Phys. Chem. B, 2010, 114, 9653–9662 CrossRef CAS PubMed.
- S. R. Meech, Chem. Soc. Rev., 2009, 38, 2922–2934 RSC.
- (a) J. S. Wu, W. M. Liu, J. C. Ge, H. Y. Zhang and P. F. Wang, Chem. Soc. Rev., 2011, 40, 3483–3495 RSC; (b) S. Goswami, A. Manna, S. Paul, A. K. Das, P. K. Nandi, A. K. Maity and P. Saha, Tetrahedron Lett., 2014, 55, 490–494 CrossRef CAS; (c) S. Goswami, A. K. Das, A. Manna, A. K. Maity, H. K. Fun, C. K. Quah and P. Saha, Tetrahedron Lett., 2014, 55, 2633–2638 CrossRef CAS; (d) S. Goswami, A. Manna, S. Paul, A. K. Das, K. Aich and P. K. Nandi, Chem. Commun., 2013, 49, 2912–2914 RSC; (e) S. Goswami, A. Manna, M. Mondal and D. Sarkar, RSC Adv., 2014, 4, 62639–62643 RSC; (f) A. Manna and S. Goswami, New J. Chem., 2015, 39, 4424–4429 RSC.
- S. Park, J. E. Kwon, S. H. Kim, J. Seo, K. Chung, S. Y. Park, D. J. Jang, B. M. Medina, J. Gierschner and S. Y. Park, J. Am. Chem. Soc., 2009, 131, 14043–14049 CrossRef CAS PubMed.
- S. Park, S. Kim, J. Seo and S. Y. Park, Macromol. Res., 2008, 16, 385–395 CrossRef CAS.
- R. R. Gagne, C. L. Spiro, T. J. Smith, C. A. Hamann, W. R. Thies and A. K. Schiemke, J. Am. Chem. Soc., 1981, 103, 4073–4081 CrossRef CAS.
- C. R. Lohani, J. M. Kim, S. Y. Chung, J. Yoon and K. H. Lee, Analyst, 2010, 135, 2079–2084 RSC.
- M. J. Frisch, et al., Gaussian 03, Revision C.02, ed. C. Gonzalez and J. A. Pople, Gaussian Inc., Wallingford, CT, 2004 Search PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 299–310 CrossRef CAS.
- V. Barone, M. Cossi and J. Tomasi, J. Comput. Chem., 1998, 19, 404–417 CrossRef CAS.
- M. Cossi, N. Rega, G. Scalmani and V. Barone, J. Comput. Chem., 2003, 24, 669–681 CrossRef CAS PubMed.
- T. Liu, H. X. Zhang and B. H. Xia, J. Phys. Chem. A, 2007, 111, 8724–8730 CrossRef CAS PubMed.
- T. Mossman, J. Immunol. Methods, 1983, 65, 55–63 CrossRef.
- S. Scheiner, J. Phys. Chem. A, 2000, 104, 5898–5909 CrossRef CAS.
- A. Weller, Elektrochemie, 1956, 60, 1144–1147 CAS.
- Fundamentals of Analytical Chemistry, ed. D. A. Skoog, D. M. West and F. J. Holler, Harcourt College Publishers, 7th edn, 1996 Search PubMed.
- C. F. Bernasconi, M. Ali and J. C. Gunter, J. Am. Chem. Soc., 2003, 125, 151–157 CrossRef CAS PubMed.
- C. F. Bernasconi, M. Ali and F. Lu, J. Am. Chem. Soc., 2000, 122, 1352–1359 CrossRef CAS.
- E. Kimura, I. Nakamura, T. Loike, M. Shionoya, Y. Kodama, T. Ikeda and M. Shiro, J. Am. Chem. Soc., 1994, 116, 4764–4771 CrossRef CAS.
- F. G. Bordwell, R. J. McCallum and W. N. Olmstead, J. Org. Chem., 1984, 49, 1424–1427 CrossRef CAS.
- T. Mistri, M. Dolai, D. Chakraborty, A. R. Khuda-Bukhsh, K. K. Das and M. Ali, Org. Biomol. Chem., 2012, 10, 2380–2384 CAS.
- J. Y. Choi, D. Kimb and J. Yoon, Dyes Pigm., 2013, 96, 176–179 CrossRef CAS.
- X. Zhou, B. Yu, Y. Guo, X. Tang, H. Zhang and W. Liu, Inorg. Chem., 2010, 49, 4002–4007 CrossRef CAS PubMed.
- S. Anbu, R. Ravishankaran, M. F. C. G. da Silva, A. A. Karande and A. J. L. Pombeiro, Inorg. Chem., 2014, 53, 6655–6664 CrossRef CAS PubMed.
- P. Roy, K. Dhara, M. Manassero, J. Ratha and P. Banerjee, Inorg. Chem., 2007, 46, 6405–6412 CrossRef CAS PubMed.
- R. Alam, T. Mistri, A. Katarkar, K. Chaudhuri, S. K. Mandal, A. R. Khuda-Bukhsh, K. K. Das and M. Ali, Analyst, 2014, 139, 4022–4030 RSC.
- B. K. Datta, D. Thiyagarajan, S. Samanta, A. Ramesh and G. Das, Org. Biomol. Chem., 2014, 12, 4975–4982 CAS.
- A. Jana, P. K. Sukul, S. K. Mandal, S. Konar, S. Ray, K. Das, J. A. Golen, A. L. Rheingold, S. Mondal, T. K. Mondal, A. R. Khuda-Bukhsh and S. K. Kar, Analyst, 2014, 139, 495–504 RSC.
- G. Buncic, P. S. Donnelly, B. M. Paterson, J. M. White, M. Zimmermann, Z. Xiao and A. G. Wedd, Inorg. Chem., 2010, 49, 3071–3073 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra25653d |
| This journal is © The Royal Society of Chemistry 2016 |