Metal-templated synthesis of intertwined, functionalized strands as precursors to molecularly woven materials†
Natasha R. Wadhwa,
Neil C. Hughes,
Jaafar A. Hachem and
Gellert Mezei*
Department of Chemistry, Western Michigan University, Kalamazoo, MI 49008, USA. E-mail: gellert.mezei@wmich.edu
First published on 22nd January 2016
Abstract
Herein we propose a novel approach toward yet to be realized molecularly woven materials (MWMs), based on metal-templated precursors containing intertwined strands with functional ends. Two different potential precursors, based on terminal alkene-functionalized bis-Schiff base ligands (with either 1,2-diaminophenylene or 1,2-diaminoethylene cores) coordinated around a Cu(I) ion, have been tested. During this work, four novel organic ligands were prepared, along with the Cu(I) or Ag(I) complexes of three of them, and were characterized by X-ray diffraction and/or NMR spectroscopy. Chemical reactivity and structural studies (by single-crystal X-ray crystallography) of these novel compounds led to the assessment of their viability as precursors for MWMs. The essential requirement that terminal functionalities on the two intertwined ligand strands of the precursor must be far enough from each other so that only inter- and no intra-molecular reactivity is possible, is only met by the precursor with a 1,2-diaminophenylene core. This precursor, however, is less stable than the analogous one with a 1,2-diaminoethylene core, as it easily undergoes intramolecular cyclization/aromatization to yield a stable benzimidazole moiety, resulting in breakdown of the strands. The benzimidazole-containing compound offers an interesting example of chiral crystallization (helical arrangement about a four-fold axis) induced by hydrogen bonding of an otherwise achiral molecule. The results of this study outline the challenges involved in the preparation of a MWM using our approach, and will aid in identifying more robust ligand systems that meet the requirements of a MWM precursor set forth here.
1. Introduction
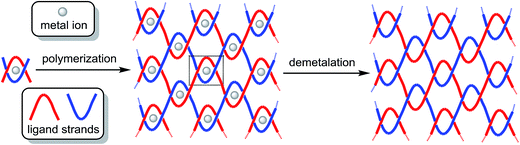
Strength, lightness and flexibility are crucial features of some materials used in everyday life. For certain specialized applications, extreme strength is required, while lightness and flexibility are still desirable. The challenge to combine these properties into one material is not trivial, as very tough materials are usually brittle and/or dense. By analogy to macroscopic woven materials, such as textiles and ropes, we predict that covalent polymers woven at the molecular level will offer the ultimate strength in a lightweight and flexible material. Mechanical failure of common polymers can occur by the disentangling of individual strands as a result of breaking weak intermolecular bonds. In contrast, disrupting a molecularly woven polymer would require the simultaneous breaking of multiple, strong covalent bonds. Although crosslinking is known to increase strength, high crosslink densities cause polymers to become rigid and glassy.1 Ultra-high molecular weight polyethylene has high impact strength, but melts at relatively low temperatures and easily deforms permanently under tensile load.2 Graphene3 and carbon nanotubes4 have the highest known tensile strengths; however, they are also the stiffest materials known and have poor resistance to bending, torsion and compression. The interlocked (but not cross-linked) strands of a molecularly woven polymer would provide inherent flexibility, lightness and extreme strength at the same time.No molecularly woven materials (MWMs) have been reported to date, as no technology is currently available for the weaving of individual polymer strands.5 The possibility of creating a “molecular macramé” by molecular weaving was anticipated in 1992 by Daryle H. Busch: a tetranuclear grid complex, with intertwined strands could, in principle, be extended in two dimensions to yield a 2D woven structure (Fig. 1).6 Although such tetranuclear precursors have been reported in the meantime,7 it is hard to imagine that long, polymeric analogs of the ligands used as strands would lead to the desired, extended woven materials. Instead, if the eight endings of the tetranuclear precursor's strands were appropriately functionalized, polymerization (“molecular sewing”) could then yield a polymer with interwoven strands. Metal complexes containing intertwined strands with functional end-groups have indeed been used for the creation of discreet catenanes, rotaxanes, knots, and higher order links.8 Other methods that could possibly be extended to molecular fabrics, such as single-stranded DNA weaving9 and surface templated synthesis,10 have been entertained.
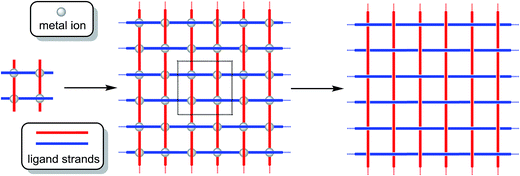 | ||
| Fig. 1 Concept of molecular weaving templated by metal ions.6 | ||
Herein we propose a simpler approach to MWMs, based on mononuclear complexes as precursors (Fig. 2), and report terminal alkene functionalized precursors that could be “sewn” together on the molecular level by alkene metathesis. Alkene metathesis11 has been used successfully for the synthesis of macrocycles,12 topologically unusual molecules (such as catenanes,13 rotaxanes14 and knots15) and polymeric materials.16 To avoid the formation of a [2]catenane (Fig. 3a) and to ensure that only inter-molecular reactivity is possible, our MWM precursor has the alkene functionalities placed close to the intertwined core and far from each other (Fig. 3b). Thus, intra-molecular reactivity is precluded based on geometric constraints.
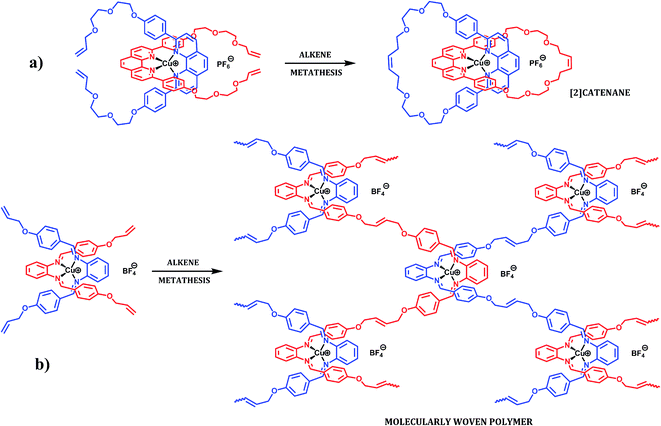 | ||
| Fig. 3 (a) Alkene functionalities in close proximity react intra-molecularly and lead to a [2]catenane (92% isolated yield);13a (b) distant alkene functionalities cannot react intra-molecularly and are expected to lead to a molecularly woven polymer. | ||
2. Results and discussion
2.1. Synthesis
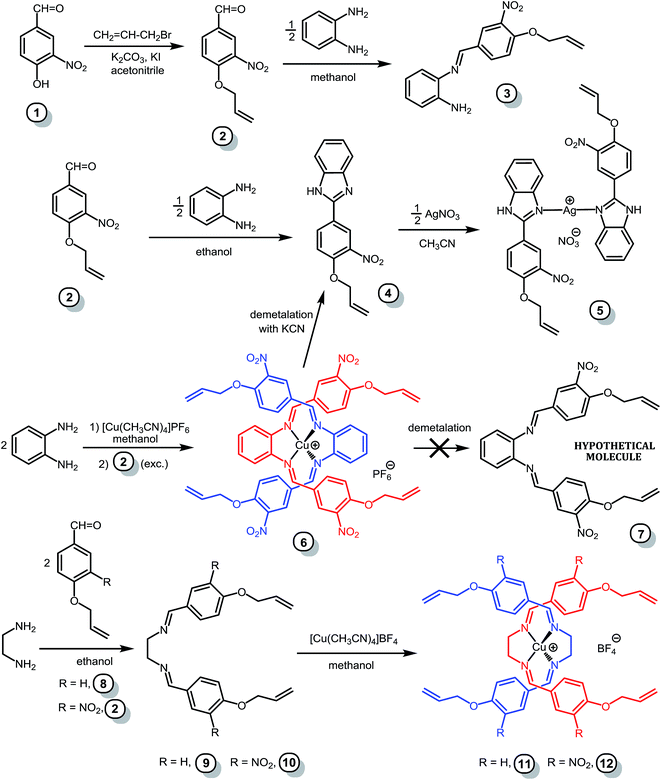
To prepare a MWM precursor with intertwined, alkene-functionalized strands, we first set out to prepare the bis-Schiff base ligand 1,2-phenylene-bis[nitrilo(E)methylidyne(4-allyloxyphenyl)] (compound 7 without the NO2-groups in Fig. 4), by the reaction of 1,2-phenylenediamine with 4-allyloxybenzaldehyde. As 1,2-phenylenediamine turned out to be unreactive toward 4-allyloxybenzaldehyde, we activated the aldehyde by attaching a nitro group to the benzene ring. 3-Nitro-4-hydroxybenzaldehyde is indeed much more reactive, so much so that during the reaction with allyl bromide, condensation of the aldehyde group with the acetone solvent also occurred (which was not the case in the absence of the NO2-group). To avoid the formation of the undesired 4-(3-nitro-4-allyloxyphenyl)-3-buten-2-one (1) (Fig. 4), acetonitrile was used instead of acetone as solvent, and 3-nitro-4-allyloxybenzaldehyde (2) was obtained in excellent yield. On reacting with 1,2-phenylenediamine (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
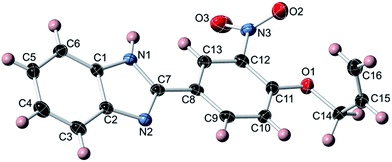
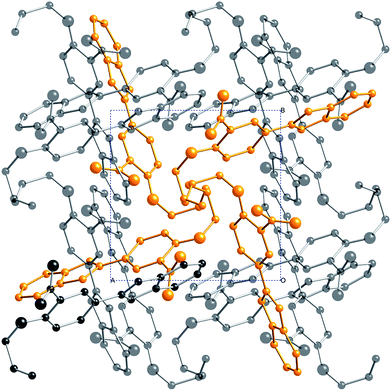
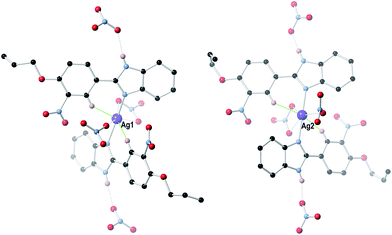
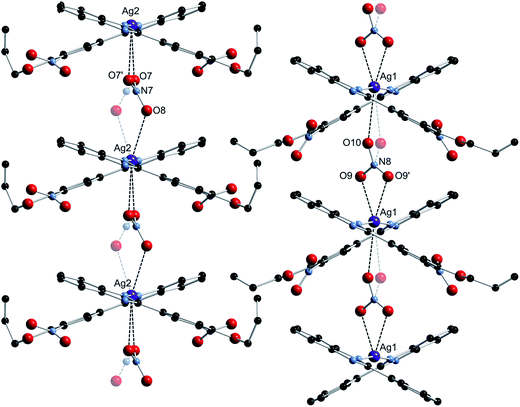
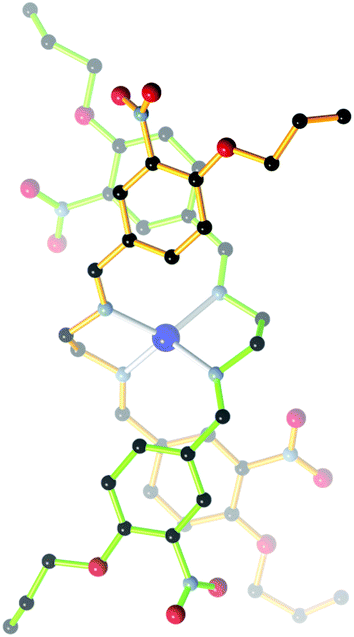
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) in methanol, 2 afforded the mono-Schiff base N-[(3-nitro-4-allyloxyphenyl)methylene]-1,2-benzenediamine (3), not the expected bis-Schiff base 7. When the same reaction was carried out in ethanol instead of methanol, 2-(3-nitro-4-allyloxyphenyl)-benzimidazole (4) was obtained instead of 7. Compound 4, an achiral molecule, was found to crystallize in a chiral space group (see the Crystallographic studies section below), and formed bis[2-(3-nitro-4-allyloxyphenyl)-benzimidazole] silver(I) nitrate (5) upon reaction with AgNO3 in acetonitrile.
1 molar ratio) in methanol, 2 afforded the mono-Schiff base N-[(3-nitro-4-allyloxyphenyl)methylene]-1,2-benzenediamine (3), not the expected bis-Schiff base 7. When the same reaction was carried out in ethanol instead of methanol, 2-(3-nitro-4-allyloxyphenyl)-benzimidazole (4) was obtained instead of 7. Compound 4, an achiral molecule, was found to crystallize in a chiral space group (see the Crystallographic studies section below), and formed bis[2-(3-nitro-4-allyloxyphenyl)-benzimidazole] silver(I) nitrate (5) upon reaction with AgNO3 in acetonitrile.
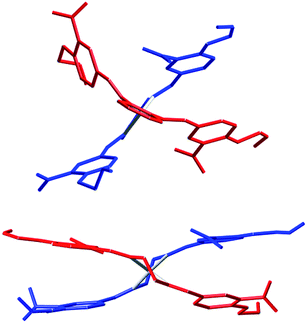
In contrast to the metal-free approach to ligand 7, the use of Cu(I) ion as template yielded completely different results. Thus, when 1,2-phenylenediamine was first reacted with [Cu(CH3CN)4]PF6 in methanol before adding 3-nitro-4-allyloxybenzaldehyde (2) in excess, the desired bis-Schiff base 7 was obtained in the form of bis[1,2-phenylenebis{nitrilo(E)methylidyne(3-nitro-4-allyloxyphenyl)}]copper(I) hexafluorophosphate (6). Crystals of 6 were obtained by slow diffusion of diethyl ether vapor into acetone or acetonitrile solutions. However, 6 turns out to be a rather delicate molecule: attempts to crash it out from an acetone solution by pouring into excess diethyl ether led to decomposition and yielded the benzimidazole 4. The same result was obtained upon attempting to prepare the free ligand 7 by demetalating 6 in acetonitrile with aqueous KCN. The cause of the observed reactivity appears to be the higher stability of benzimidazole due to extended aromaticity.
Although metal-free 1,2-diaminophenylene-based bis-Schiff base ligands can undoubtedly be obtained,17 it appears that the presence of phenyl groups on the methine carbons leads exclusively to the formation of 2-phenylbenzimidazoles and/or 1-benzyl-2-phenylbenzimidazoles.18 A notable exception is the case of phenyl groups with an ortho-OH19 or NH20 group, which appear to stabilize the free bis-Schiff base ligand. Coordination to Cu(I) ions stabilizes the elusive ligand 7, and provides the only known example of a 1,2-diaminophenylene-based bis-Schiff base (6) without a stabilizing ortho-OH or NH group on the methine-bound phenyl groups.
Based on the fact that ligand 7 could not be obtained in the metal-free form, we predict that demetalation of a MWM based on 6 will lead to breakdown of the material, as the more stable benzimidazole moiety forms upon rupture of the strands. Therefore, we next targeted analogous ligands, with 1,2-diaminoethylene instead of 1,2-diaminophenylene core, by reacting ethylenediamine with 4-allyloxybenzaldehyde (8) or its nitro derivative (2). Unlike in the case of the 1,2-diaminophenylene core, the corresponding bis-Schiff bases 9 and 10, which cannot form benzimidazoles, were readily obtained (Fig. 4). Bis-Schiff bases 9 and 10 were then reacted with [Cu(CH3CN)4]BF4 in methanol to yield the MWM precursors 11 and 12. Differences between the structures of 6 and 12, caused by changing the 1,2-diaminophenylene core to a 1,2-diaminoethylene core, are discussed below in the ‘Crystallographic studies’ section.
2.2. Crystallographic studies
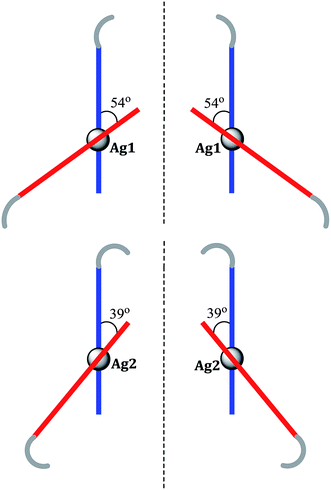 | ||
| Fig. 8 Schematic representation of the four different conformers found in the crystal structure of 5, showing the differences in allyl group orientations and in angles between ligand strands. | ||
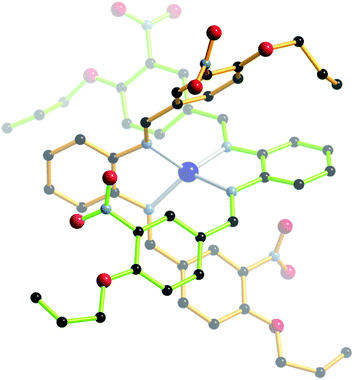 | ||
| Fig. 10 Ball-and-stick representation of the crystal structure of 6 (PF6− counterion and H-atoms omitted for clarity). | ||
3. Summary
Herein we have set forth a novel approach toward the preparation of hitherto elusive molecularly woven materials, by “sewing” together (polymerizing) precursors with intertwined, functionalized strands. An essential feature of these precursors is the placement of the terminal functionalities so that only inter- and no intra-molecular reactivity is possible. We have prepared precursors based on terminal alkene-functionalized bis-Schiff base ligands (with either a 1,2-diaminophenylene or a 1,2-diaminoethylene core), coordinated around a Cu(I) ion. It first became evident that the bis-Schiff base with the 1,2-diaminophenylene core (7) can easily undergo intramolecular cyclization/aromatization to yield a stable benzimidazole moiety. Therefore, 7 could only be isolated as the tetrahedral Cu(I) complex (6). The analogous bis-Schiff bases with a 1,2-diaminoethylene core (9 and 10), in turn, are stable in both metal-free and complex forms. The structure of the two different precursors, derived from X-ray crystallographic studies, reveals another crucial requirement for the MWM precursor. While in 6 the rigid 1,2-diaminophenylene cores do orient the strands so that the terminal alkene functionalities are far from each other, in 12 the flexible 1,2-diaminoethylene core allows the functional groups to approach each other close enough for intramolecular reactions to possibly occur. Furthermore, it also becomes evident that although 6 and 12 are perhaps the synthetically most easily accessible and inexpensive candidates, they are inadequate as MWM precursors, as during alkene metathesis, (PCy3)2Cl2Ru![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh (Grubbs catalyst) consistently led to breakdown of the bis-Schiff base ligand (to the benzimidazole derivative 4 in the case of 6, and to the starting material 2 in the case of 12). We are currently targeting more robust ligand systems that will address all the requirements for a MWM precursor revealed by this work.
CHPh (Grubbs catalyst) consistently led to breakdown of the bis-Schiff base ligand (to the benzimidazole derivative 4 in the case of 6, and to the starting material 2 in the case of 12). We are currently targeting more robust ligand systems that will address all the requirements for a MWM precursor revealed by this work.
4. Experimental section
4.1. General
Acetone was dried with CaSO4, distilled under N2 and stored over 4 Å molecular sieves. 4-Allyloxybenzaldehyde,25 [Cu(CH3CN)4]BF4 and [Cu(CH3CN)4]PF6 (ref. 26) were prepared according to the literature. 3-Nitro-4-hydroxybenzaldehyde was obtained by the nitration of 4-hydroxybenzaldehyde using a modified procedure from the literature.27 1,2-Benzenediamine (o-phenylenediamine) was recrystallized from boiling water/activated charcoal. K2CO3 and KI were dried overnight in an oven at 130 °C. All other commercially available reagents were used as received. NMR spectra were recorded on a Jeol JNM-ECP400 spectrometer.4.2. Synthesis of 3-nitro-4-hydroxybenzaldehyde (1)
As the published procedure27 was found to yield only 15–22% of product, an improved procedure is reported herein. 4-Hydroxybenzaldehyde (12.0 g, 0.0983 mol) was dissolved with stirring in 100 mL glacial acetic acid, and the solution was cooled in an ice bath. Under vigorous stirring, HNO3 (70%) (35 mL, 35 g pure HNO3, 0.55 mol) was added dropwise over 15 minutes. After stirring for 20 hours (the ice slowly melted and the solution warmed up to room temperature), the reaction mixture was cooled in an ice bath. The solid was filtered out, washed with two 7 mL portions of ice-cold acetic acid (70% in water) and then with six 25 mL portions of water. After drying in air, the product was kept in high vacuum for 12 hours. Yield: 8.38 g (51%). 1H NMR (400 MHz, CDCl3): 11.01 (s, 1H, OH), 9.93 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 8.62 (d, 1H, 4J = 2.2 Hz), 8.13 (dd, 1H, 3J = 8.8 Hz, 4J = 2.2 Hz), 7.31 (d, 1H, 3J = 8.8 Hz).
O), 8.62 (d, 1H, 4J = 2.2 Hz), 8.13 (dd, 1H, 3J = 8.8 Hz, 4J = 2.2 Hz), 7.31 (d, 1H, 3J = 8.8 Hz).
4.3. Synthesis of 3-nitro-4-allyloxybenzaldehyde (2)
3-Nitro-4-hydroxybenzaldehyde (0.55 g, 3.3 mmol), anhydrous K2CO3 (1.40 g, 10.1 mmol), KI (0.133 g, 0.80 mmol) and allyl bromide (0.90 mL, 1.26 g, 10.4 mmol) were refluxed in 50 mL anhydrous acetonitrile under an N2 atmosphere for 20 hours. After removal of the solvent and excess allyl bromide in vacuum, the residue was dissolved in 100 mL chloroform and the solution was filtered to remove the insoluble inorganic salts. Evaporation of the solvent in vacuum yielded 0.66 g of 2 as a yellow liquid, which slowly solidified on standing. Yield: 97%. 1H NMR (400 MHz, CDCl3): 9.92 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 8.34 (d, 1H, 4J = 1.8 Hz), 8.05 (dd, 1H, 3J = 8.8 Hz, 4J = 2.2 Hz), 7.21 (d, 1H, 3J = 8.4 Hz), 6.03 (m, 1H), 5.51 (dm, 1H, 3J = 17.6 Hz), 5.38 (dm, 1H, 3J = 11.7 Hz), 4.79 (m, 2H) ppm. 13C NMR (101 MHz, CDCl3): 189.2, 156.0, 139.8, 135.1, 131.0, 129.0, 126.8, 118.8, 115.1, 70.4 ppm.
O), 8.34 (d, 1H, 4J = 1.8 Hz), 8.05 (dd, 1H, 3J = 8.8 Hz, 4J = 2.2 Hz), 7.21 (d, 1H, 3J = 8.4 Hz), 6.03 (m, 1H), 5.51 (dm, 1H, 3J = 17.6 Hz), 5.38 (dm, 1H, 3J = 11.7 Hz), 4.79 (m, 2H) ppm. 13C NMR (101 MHz, CDCl3): 189.2, 156.0, 139.8, 135.1, 131.0, 129.0, 126.8, 118.8, 115.1, 70.4 ppm.
4.4. Synthesis of N-[(3-nitro-4-allyloxyphenyl)methylene]-1,2-benzenediamine (3)
A solution of 1,2-benzenediamine (0.058 g, 0.54 mmol) in 3.3 mL methanol was added dropwise to a solution of 2 (0.330 g, 1.59 mmol) in 1.6 mL methanol, and the mixture was stirred for two days. The yellow precipitate was filtered, washed with methanol and dried in vacuum. Yield: 0.089 g (68%). 1H NMR (400 MHz, CDCl3): 8.48 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 8.39 (d, 1H, 4J = 2.2 Hz), 8.04 (dd, 1H, 3J = 8.8 Hz, 4J = 2.2 Hz), 7.14 (d, 1H, 3J = 8.8 Hz), 7.07 (m, 2H), 6.76 (m, 2H), 6.05 (m, 1H), 5.52 (dm, 1H, 3J = 17.2 Hz), 5.37 (dm, 1H, 3J = 10.6 Hz), 4.76 (m, 2H), 4.25 (s, 2H, NH2) ppm.
CH), 8.39 (d, 1H, 4J = 2.2 Hz), 8.04 (dd, 1H, 3J = 8.8 Hz, 4J = 2.2 Hz), 7.14 (d, 1H, 3J = 8.8 Hz), 7.07 (m, 2H), 6.76 (m, 2H), 6.05 (m, 1H), 5.52 (dm, 1H, 3J = 17.2 Hz), 5.37 (dm, 1H, 3J = 10.6 Hz), 4.76 (m, 2H), 4.25 (s, 2H, NH2) ppm.
4.5. Synthesis of 2-(3-nitro-4-allyloxyphenyl)-benzimidazole (4)
A solution of 1,2-benzenediamine (0.17 g, 1.6 mmol) in 2.5 mL absolute ethanol was stirred overnight with a solution of 2 (0.66 g, 3.2 mmol) in 5 mL absolute ethanol. The yellow precipitate was filtered, washed with a small amount of ethanol and dried in vacuum. Recrystallization from acetone provided 0.32 g (68%) of 4 as light yellow crystals. 1H NMR (400 MHz, acetone-d6): 12.06 (s, 1H), 8.65 (d, 1H, 4J = 1.4 Hz), 8.44 (dd, 1H, 3J = 8.8 Hz, 4J = 1.5 Hz), 7.68 (m, 1H), 7.52 (m, 1H), 7.51 (d, 1H, 3J = 8.8 Hz), 7.22 (m, 2H), 6.11 (m, 1H), 5.53 (dm, 1H, 3J = 17.1 Hz), 5.32 (dm, 1H, 3J = 10.8 Hz), 4.87 (m, 2H) ppm. 13C NMR (101 MHz, acetone-d6): 152.4, 149.4, 144.4, 140.3, 135.3, 132.3, 131.9, 123.4, 123.1, 123.0, 122.1, 119.4, 117.6, 115.8, 111.2, 70.0 ppm. Pale-yellow X-ray quality single-crystals were obtained by slow cooling of a concentrated, hot acetone solution.4.6. Synthesis of bis[2-(3-nitro-4-allyloxyphenyl)-benzimidazole]silver(I) nitrate (5)
A solution of 4 (14 mg, 53 μmol) in 3 mL CH3CN was layered over a solution of AgNO3 (2.6 mg, 15 μmol) in 1 mL CH3CN. After a few days yellow X-ray quality single-crystals of 5 were obtained. 1H NMR (400 MHz, DMSO-d6): 13.22 (s, 2H), 8.68 (d, 2H, 4J = 2.2 Hz), 8.42 (dd, 2H, 3J = 8.8 Hz, 4J = 2.2 Hz), 7.69 (d, 2H, 3J = 7.4 Hz), 7.56 (m, 4H), 7.24 (m, 4H), 6.06 (m, 2H), 5.48 (dd, 2H, 2J = 1.1 Hz, 3J = 17.2 Hz), 5.33 (dd, 2H, 2J = 1.1 Hz, 3J = 10.6 Hz), 4.87 (d, 4H, 3J = 5.1 Hz) ppm. 13C NMR (101 MHz, DMSO-d6): 152.6, 149.9, 140.1, 132.9, 132.8, 123.7, 123.1, 118.7, 118.6, 116.7, 70.3 ppm.4.7. Synthesis of bis[1,2-phenylenebis{nitrilo(E)methylidyne(3-nitro-4-allyloxyphenyl)}]copper(I) hexafluorophosphate (6)
A colorless solution of [Cu(CH3CN)4]PF6 (0.302 g, 0.810 mmol) in 15 mL methanol was added under an N2 atmosphere to a solution of 1,2-benzenediamine (0.178 g, 1.65 mmol) in 5 mL methanol. The purple solution obtained was transferred into a solution of 3-nitro-4-allyloxybenzaldehyde (1.000 g, 4.83 mmol) in 10 mL methanol under N2. A bright red precipitate was obtained, which was stirred for 24 hours, filtered, washed with methanol and dried in vacuum. 0.626 g of 5 was obtained as a red powder. Yield: 65%. 1H NMR (400 MHz, acetone-d6): 8.95 (s, 4H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 8.30 (d, 4H, 4J = 1.8 Hz), 8.02 (dd, 4H, 3J = 8.8 Hz, 4J = 1.8 Hz), 7.69 (s, 8H), 6.93 (d, 4H, 3J = 8.8 Hz), 6.01 (m, 4H), 5.43 (dm, 4H, 3J = 17.2 Hz), 5.31 (dm, 4H, 3J = 10.6 Hz), 4.73 (m, 8H) ppm. 13C NMR (101 MHz, CDCl3): 161.5, 154.5, 144.1, 139.3, 135.2, 131.8, 129.9, 126.9, 126.1, 120.2, 117.8, 115.1, 70.2 ppm. Red X-ray quality single-crystals were grown from acetone or acetonitrile solution by slow diethyl ether vapor diffusion.
CH), 8.30 (d, 4H, 4J = 1.8 Hz), 8.02 (dd, 4H, 3J = 8.8 Hz, 4J = 1.8 Hz), 7.69 (s, 8H), 6.93 (d, 4H, 3J = 8.8 Hz), 6.01 (m, 4H), 5.43 (dm, 4H, 3J = 17.2 Hz), 5.31 (dm, 4H, 3J = 10.6 Hz), 4.73 (m, 8H) ppm. 13C NMR (101 MHz, CDCl3): 161.5, 154.5, 144.1, 139.3, 135.2, 131.8, 129.9, 126.9, 126.1, 120.2, 117.8, 115.1, 70.2 ppm. Red X-ray quality single-crystals were grown from acetone or acetonitrile solution by slow diethyl ether vapor diffusion.
4.8. Synthesis of 1,2-ethylenebis[nitrilo(E)methylidyne(4-allyloxyphenyl)] (9)
A solution of ethylenediamine (0.760 g, 12.6 mmol) in 5 mL absolute ethanol was added dropwise to a solution of 4-allyloxybenzaldehyde (4.010 g, 24.7 mmol) in 35 mL absolute ethanol. A white precipitate started forming soon, which was stirred overnight (∼18 h), then filtered out, washed with absolute ethanol and dried in vacuum. Yield: 3.25 g (75%). 1H NMR (400 MHz, CDCl3): 8.19 (s, 2H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.61 (d, 4H, 3J = 8.8 Hz), 6.90 (d, 4H, 3J = 8.8 Hz), 6.04 (m, 2H), 5.40 (dm, 2H, 3J = 17.3 Hz), 5.29 (dm, 2H, 3J = 10.3 Hz), 4.55 (dm, 4H, 3J = 5.4 Hz), 3.90 (s, 4H) ppm. 13C NMR (101 MHz, CDCl3): 162.0, 160.6, 133.0, 129.7, 129.3, 118.0, 114.8, 68.9, 61.8 ppm. 1H NMR (400 MHz, acetone-d6): 8.23 (s, 2H, N
CH), 7.61 (d, 4H, 3J = 8.8 Hz), 6.90 (d, 4H, 3J = 8.8 Hz), 6.04 (m, 2H), 5.40 (dm, 2H, 3J = 17.3 Hz), 5.29 (dm, 2H, 3J = 10.3 Hz), 4.55 (dm, 4H, 3J = 5.4 Hz), 3.90 (s, 4H) ppm. 13C NMR (101 MHz, CDCl3): 162.0, 160.6, 133.0, 129.7, 129.3, 118.0, 114.8, 68.9, 61.8 ppm. 1H NMR (400 MHz, acetone-d6): 8.23 (s, 2H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.67 (d, 4H, 3J = 8.8 Hz), 6.96 (d, 4H, 3J = 8.8 Hz), 6.06 (m, 2H), 5.41 (dm, 2H, 3J = 17.2 Hz), 5.25 (dm, 2H, 3J = 10.6 Hz), 4.60 (dm, 4H, 3J = 5.2 Hz), 3.83 (s, 4H) ppm. 13C NMR (101 MHz, acetone-d6): 160.9, 160.7, 133.6, 129.8, 129.5, 116.8, 114.6, 68.5, 61.7 ppm.
CH), 7.67 (d, 4H, 3J = 8.8 Hz), 6.96 (d, 4H, 3J = 8.8 Hz), 6.06 (m, 2H), 5.41 (dm, 2H, 3J = 17.2 Hz), 5.25 (dm, 2H, 3J = 10.6 Hz), 4.60 (dm, 4H, 3J = 5.2 Hz), 3.83 (s, 4H) ppm. 13C NMR (101 MHz, acetone-d6): 160.9, 160.7, 133.6, 129.8, 129.5, 116.8, 114.6, 68.5, 61.7 ppm.
4.9. Synthesis of 1,2-ethylenebis[nitrilo(E)methylidyne(3-nitro-4-allyloxyphenyl)] (10)
A solution of ethylenediamine (0.680 g, 11.3 mmol) in 5 mL absolute ethanol was added dropwise to a slightly warmed (30–40 °C) solution of 3-nitro-4-allyloxybenzaldehyde (4.500 g, 21.7 mmol) in 40 mL absolute ethanol. Within an hour a large amount of precipitate formed. After stirring overnight (∼18 h), the yellow precipitate was filtered out, washed with absolute ethanol and dried in vacuum. Yield: 3.58 g (75%). 1H NMR (400 MHz, CDCl3): 8.20 (s, 2H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 8.15 (d, 2H, 4J = 2.2 Hz), 7.84 (dd, 2H, 3J = 8.4 Hz, 4J = 2.2 Hz), 7.06 (d, 2H, 3J = 8.4 Hz), 6.01 (m, 2H), 5.47 (dm, 2H, 3J = 17.2 Hz), 5.33 (dm, 2H, 3J = 10.6 Hz), 4.70 (dm, 4H, 3J = 5.2 Hz), 3.94 (s, 4H) ppm. 13C NMR (101 MHz, CDCl3): 159.6, 153.4, 140.1, 133.2, 131.4, 129.1, 125.3, 118.8, 114.8, 70.2, 61.3. 13C NMR (400 MHz, CDCl3): 159.5, 153.3, 139.9, 133.4, 131.4, 129.1, 125.0, 118.6, 114.8, 70.1, 61.3 ppm. 1H NMR (400 MHz, acetone-d6): 8.34 (s, 2H, N
CH), 8.15 (d, 2H, 4J = 2.2 Hz), 7.84 (dd, 2H, 3J = 8.4 Hz, 4J = 2.2 Hz), 7.06 (d, 2H, 3J = 8.4 Hz), 6.01 (m, 2H), 5.47 (dm, 2H, 3J = 17.2 Hz), 5.33 (dm, 2H, 3J = 10.6 Hz), 4.70 (dm, 4H, 3J = 5.2 Hz), 3.94 (s, 4H) ppm. 13C NMR (101 MHz, CDCl3): 159.6, 153.4, 140.1, 133.2, 131.4, 129.1, 125.3, 118.8, 114.8, 70.2, 61.3. 13C NMR (400 MHz, CDCl3): 159.5, 153.3, 139.9, 133.4, 131.4, 129.1, 125.0, 118.6, 114.8, 70.1, 61.3 ppm. 1H NMR (400 MHz, acetone-d6): 8.34 (s, 2H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 8.19 (d, 2H, 4J = 1.8 Hz), 7.96 (dd, 2H, 3J = 8.8 Hz, 4J = 1.8 Hz), 7.37 (d, 2H, 3J = 8.8 Hz), 6.07 (m, 2H), 5.48 (dm, 2H, 3J = 17.6 Hz), 5.29 (dm, 2H, 3J = 10.6 Hz), 4.82 (dm, 4H, 3J = 5.1 Hz), 3.92 (s, 4H) ppm. 13C NMR (101 MHz, acetone-d6): 159.4, 152.8, 140.4, 133.2, 132.3, 129.6, 124.0, 117.5, 115.2, 69.9, 61.1 ppm.
CH), 8.19 (d, 2H, 4J = 1.8 Hz), 7.96 (dd, 2H, 3J = 8.8 Hz, 4J = 1.8 Hz), 7.37 (d, 2H, 3J = 8.8 Hz), 6.07 (m, 2H), 5.48 (dm, 2H, 3J = 17.6 Hz), 5.29 (dm, 2H, 3J = 10.6 Hz), 4.82 (dm, 4H, 3J = 5.1 Hz), 3.92 (s, 4H) ppm. 13C NMR (101 MHz, acetone-d6): 159.4, 152.8, 140.4, 133.2, 132.3, 129.6, 124.0, 117.5, 115.2, 69.9, 61.1 ppm.
4.10. Synthesis of bis[1,2-ethylenebis{nitrilo(E)methylidyne(4-allyloxyphenyl)}]copper(I) tetrafluoroborate (11)
[Cu(CH3CN)4]BF4 (0.225 g, 0.715 mmol) in 7.5 mL CH3CN was added to compound 9 (0.500 g, 1.43 mmol) in 30 mL CH3CN under stirring. The colorless solution immediately turned orange. After stirring for 10 minutes, the solvent was removed in vacuum. Yield: 0.59 g (97%). 1H NMR (400 MHz, CDCl3): 8.27 (s, 2H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.60 (d, 4H, 3J = 8.8 Hz), 6.84 (d, 4H, 3J = 8.8 Hz), 6.02 (m, 2H), 5.40 (dm, 2H, 3J = 17.6 Hz), 5.28 (dm, 2H, 3J = 10.6 Hz), 4.56 (s, 4H), 3.97 (s, 4H) ppm. 1H NMR (400 MHz, acetone-D6): 8.52 (s, 2H, N
CH), 7.60 (d, 4H, 3J = 8.8 Hz), 6.84 (d, 4H, 3J = 8.8 Hz), 6.02 (m, 2H), 5.40 (dm, 2H, 3J = 17.6 Hz), 5.28 (dm, 2H, 3J = 10.6 Hz), 4.56 (s, 4H), 3.97 (s, 4H) ppm. 1H NMR (400 MHz, acetone-D6): 8.52 (s, 2H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.81 (d, 4H, 3J = 8.8 Hz), 7.00 (d, 4H, 3J = 8.8 Hz), 6.04 (m, 2H), 5.40 (dm, 2H, 3J = 17.6 Hz), 5.26 (dm, 2H, 3J = 10.6 Hz), 4.61 (dm, 4H, 3J = 5.1 Hz), 4.14 (s, 4H) ppm. 13C NMR (101 MHz, acetone-D6): 164.4, 161.7, 133.3, 130.8, 127.2, 117.1, 114.6, 68.7, 61.4 ppm.
CH), 7.81 (d, 4H, 3J = 8.8 Hz), 7.00 (d, 4H, 3J = 8.8 Hz), 6.04 (m, 2H), 5.40 (dm, 2H, 3J = 17.6 Hz), 5.26 (dm, 2H, 3J = 10.6 Hz), 4.61 (dm, 4H, 3J = 5.1 Hz), 4.14 (s, 4H) ppm. 13C NMR (101 MHz, acetone-D6): 164.4, 161.7, 133.3, 130.8, 127.2, 117.1, 114.6, 68.7, 61.4 ppm.
4.11. Synthesis of bis[1,2-ethylenebis{nitrilo(E)methylidyne(3-nitro-4-allyloxyphenyl)}]copper(I) tetrafluoroborate (12)
[Cu(CH3CN)4]BF4 (0.180 g, 0.572 mmol) in 4 mL CH3CN was added to compound 10 (0.500 g, 1.14 mmol) in 19 mL CH3CN under stirring. The color of the solution turned from yellow to orange. After stirring for 10 minutes, the solvent was removed in vacuum. Yield: 0.55 g (94%). 1H NMR (400 MHz, acetone-D6): 8.67 (s, 2H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 8.20 (d, 2H, 4J = 2.2 Hz), 7.96 (dd, 2H, 3J = 8.8 Hz, 4J = 1.8 Hz), 7.45 (d, 2H, 3J = 8.8 Hz), 6.07 (m, 2H), 5.49 (dm, 2H, 3J = 17.2 Hz), 5.33 (dm, 2H, 3J = 10.6 Hz), 4.84 (dm, 4H, 3J = 5.1 Hz), 4.19 (s, 4H) ppm. 13C NMR (101 MHz, acetone-D6): 164.1, 154.0, 139.7, 135.0, 132.1, 126.5, 124.3, 117.8, 115.5, 70.2, 61.9 ppm. Single-crystals were grown from a nitrobenzene solution by diethyl ether vapor diffusion.
CH), 8.20 (d, 2H, 4J = 2.2 Hz), 7.96 (dd, 2H, 3J = 8.8 Hz, 4J = 1.8 Hz), 7.45 (d, 2H, 3J = 8.8 Hz), 6.07 (m, 2H), 5.49 (dm, 2H, 3J = 17.2 Hz), 5.33 (dm, 2H, 3J = 10.6 Hz), 4.84 (dm, 4H, 3J = 5.1 Hz), 4.19 (s, 4H) ppm. 13C NMR (101 MHz, acetone-D6): 164.1, 154.0, 139.7, 135.0, 132.1, 126.5, 124.3, 117.8, 115.5, 70.2, 61.9 ppm. Single-crystals were grown from a nitrobenzene solution by diethyl ether vapor diffusion.
4.12. X-ray crystallography
X-ray diffraction data were collected at 100 K from a single-crystal mounted atop a glass fiber under Paratone-N oil, with a Bruker SMART APEX II diffractometer using graphite-monochromated Mo-Kα (λ = 0.71073 Å) radiation. The structures were solved by employing SHELXTL direct methods and refined by full-matrix least squares on F2, using the APEX2 v2014.9-0 software package (Bruker AXS Inc.: Madison, WI, 2014).28 All non-H atoms were refined with independent anisotropic displacement parameters. Hydrogen atoms were placed at calculated positions and refined using a riding model. Bond-length and atomic displacement parameter restraints were used for the disordered groups in 12. Crystallographic data have been deposited at the Cambridge Crystallographic Data Centre (deposition numbers: CCDC 1439669–1439672). Crystallographic details are summarized in Table 1.| Compound | 4 | 5 | 6 | 12 C6H5NO2 |
| Formula | C16H13N3O3 | C32H26AgN7O9 | C52H44CuF6N8O12P | C50H49BCuF4N9O14 |
| Formula weight | 295.29 | 760.47 | 1181.46 | 1150.33 |
| Crystal system | Tetragonal | Monoclinic | Monoclinic | Monoclinic |
| Space group | P43 | P2/c | P21/n | P21/n |
| a/Å | 8.7624(1) | 26.7698(3) | 15.2157(2) | 14.5499(2) |
| b/Å | 8.7624(1) | 7.3672(1) | 14.5593(2) | 18.8640(2) |
| c/Å | 18.3758(2) | 15.5539(2) | 22.9542(3) | 19.3788(2) |
| α/o | 90 | 90 | 90 | 90 |
| β/o | 90 | 97.758(1) | 102.021(1) | 105.378(1) |
| γ/o | 90 | 90 | 90 | 90 |
| V/Å3 | 1410.89(3) | 3039.44(7) | 4973.5(1) | 5128.45(11) |
| Z | 4 | 4 | 4 | 4 |
| Dcalc/g cm−3 | 1.390 | 1.662 | 1.578 | 1.490 |
| Absorption coefficient, μ/mm−1 | 0.099 | 0.734 | 0.569 | 0.517 |
| Reflections collected/unique | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 826/3510 826/3510 |
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 487/6232 487/6232 |
94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 617/11 617/11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 859 859 |
89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 433/10 433/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 493 493 |
| Observed reflections (I > 2σ(I)) | 2811 | 4468 | 8664 | 8312 |
| Goodness-of-fit (on F2) | 1.047 | 1.007 | 1.027 | 1.009 |
| R1(F); Rw(F) (I > 2σ(I)) | 0.0437; 0.0908 | 0.0389; 0.0682 | 0.0408; 0.0928 | 0.0359; 0.0779 |
| R1(F); Rw(F) (all data) | 0.0628; 0.0995 | 0.0718; 0.0778 | 0.0673; 0.1054 | 0.0524; 0.0857 |
| Largest diff. peak & hole (e Å−3) | 0.341/−0.232 | 0.463/−0.551 | 0.458/−0.508 | 0.403/−0.393 |
Acknowledgements
This material is based upon work supported by a Faculty Research and Creative Activities Award from Western Michigan University (FRACAA, No. P11-014).References
- “Engineering with Rubber”, ed. A. N. Gent, Carl Hanser Verlag, Munich, 2nd edn, 2001 Search PubMed.
- H. L. Stein, “Ultrahigh molecular weight polyethylene (UHMWPE)”, in Engineered Materials Handbook (Vol. 2: Engineering Plastics), ed. C. Dostal, ASM International, 1988, pp. 167–171 Search PubMed.
- C. Lee, X. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385–388 CrossRef CAS.
- (a) P. J. F. Harris, Carbon Nanotube Science: Synthesis, Properties and Applications, Cambridge University Press, 2009 Search PubMed; (b) A. Jorio, G. Dresselhaus and M. S. Dresselhaus, Carbon Nanotubes: Advanced Topics in the Synthesis, Structure, Properties and Applications, Springer, 2008 Search PubMed.
- Molecularly woven materials (MWMs) are defined here as covalent molecular strands that are mechanically interlocked by multiple crossings, excluding structures based on strands consisting of smaller units linked together by coordinate bonds, hydrogen bonds, or other weak bonds.
- (a) D. H. Busch, J. Inclusion Phenom. Mol. Recognit. Chem., 1992, 12, 389–395 CrossRef CAS; (b) D. H. Busch, “Ligand design for enhanced molecular organization – Selectivity and specific sequencing in multiple receptor ligands, and orderly molecular entanglements”, in Transition Metals in Supramolecular Chemistry, ed. L. Fabbrizzi and A. Poggi, Kluwer Academic Publishing, 1994, pp. 55–80 Search PubMed; (c) T. J. Hubin, A. G. Kolchinski, A. L. Vance and D. H. Busch, Adv. Supramol. Chem., 1999, 5, 237–357 CrossRef CAS; (d) T. J. Hubin and D. H. Busch, Coord. Chem. Rev., 2000, 200–202, 5–52 CrossRef CAS; (e) D. L. Cockriel, J. M. McClain, K. C. Patel, R. Ullom, T. R. Hasley, S. J. Archibald and T. J. Hubin, Inorg. Chem. Commun., 2008, 11, 1–4 CrossRef CAS.
- (a) J. Hausmann and S. Brooker, Chem. Commun., 2004, 1530–1531 RSC; (b) C. S. Campos-Fernández, R. Clérac and K. R. Dunbar, Angew. Chem., Int. Ed., 1999, 38, 3477–3479 CrossRef; (c) T. Bark, M. Düggeli, H. Stoeckli-Evans and A. von Zelewsky, Angew. Chem., Int. Ed., 2001, 40, 2848–2851 CrossRef CAS.
- (a) R. S. Forgan, J.-P. Sauvage and J. F. Stoddart, Chem. Rev., 2011, 111, 5434–5464 CrossRef CAS PubMed; (b) J. E. Beves, B. A. Blight, C. J. Campbell, D. A. Leigh and R. T. McBurney, Angew. Chem., Int. Ed., 2011, 50, 9260–9327 CrossRef CAS PubMed.
- (a) T. Ciengshin, R. Sha and N. C. Seeman, Angew. Chem., Int. Ed., 2011, 50, 4419–4422 CrossRef CAS PubMed; (b) W. Liu, H. Zhong, R. Wang and N. C. Seeman, Angew. Chem., Int. Ed., 2011, 50, 264–267 CrossRef CAS PubMed.
- D. Andrae, New J. Chem., 2006, 30, 873–882 RSC.
- (a) R. H. Grubbs, Handbook of Metathesis, Wiley-VCH, Weinheim, 2003 Search PubMed; (b) R. H. Grubbs, Tetrahedron, 2004, 60, 7117–7140 CrossRef CAS; (c) R. H. Grubbs and T. M. Trnka, “Ruthenium-catalyzed olefin metathesis”, in Ruthenium in Organic Synthesis, ed. S.-I. Murahashi, Wiley-VCH, Verlag GmbH, 2005 Search PubMed; (d) D. Astruc, New J. Chem., 2005, 29, 42–56 RSC; (e) R. R. Schrock and A. H. Hoveyda, Angew. Chem., Int. Ed., 2003, 42, 4592–4633 CrossRef CAS PubMed; (f) T. M. Trnka and R. H. Grubbs, Acc. Chem. Res., 2001, 34, 18–29 CrossRef CAS PubMed; (g) G. C. Vougioukalakis and R. H. Grubbs, Chem. Rev., 2010, 110, 1746–1787 CrossRef CAS PubMed.
- (a) C. W. Lee and R. H. Grubbs, J. Org. Chem., 2001, 66, 7155–7158 CrossRef CAS PubMed; (b) A. V. Chuchuryukin, H. P. Dijkstra, B. M. J. M. Suijkerbuijk, R. J. M. Klein Gebbink, G. P. M. van Klink, A. M. Mills, A. L. Spek and G. van Koten, Angew. Chem., Int. Ed., 2003, 115, 238–240 CrossRef; (c) M. Yu, C. Wang, A. F. Kyle, P. Jakubec, D. J. Dixon, R. R. Schrock and A. H. Hoveyda, Nature, 2011, 479, 88–93 CrossRef CAS PubMed; (d) A. Gradillas and J. Pérez-Castells, Angew. Chem., Int. Ed., 2006, 45, 6086–6101 CrossRef CAS PubMed.
- (a) B. Mohr, M. Weck, J.-P. Sauvage and R. H. Grubbs, Angew. Chem., Int. Ed., 1997, 36, 1308–1310 CrossRef CAS; (b) D. A. Leigh, P. J. Lusby, R. T. McBurney, A. Morelli, A. M. Z. Slawin, A. R. Thomson and D. B. Walker, J. Am. Chem. Soc., 2009, 131, 3762–3771 CrossRef CAS PubMed; (c) M. Gupta, S. Kang and M. F. Mayer, Tetrahedron Lett., 2008, 49, 2946–2950 CrossRef CAS; (d) E. N. Guidry, S. J. Cantrill, J. F. Stoddart and R. H. Grubbs, Org. Lett., 2005, 7, 2129–2132 CrossRef CAS PubMed; (e) H. Iwamoto, K. Itoh, H. Nagamiya and Y. Fukazawa, Tetrahedron Lett., 2003, 44, 5773–5776 CrossRef CAS; (f) F. Arico, P. Mobian, J.-M. Kern and J.-P. Sauvage, Org. Lett., 2003, 5, 1887–1890 CrossRef CAS PubMed; (g) M. Weck, B. Mohr, J.-P. Sauvage and R. H. Grubbs, J. Org. Chem., 1999, 64, 5463–5471 CrossRef CAS PubMed; (h) D. A. Leigh, R. G. Pritchard and A. J. Stephens, Nat. Chem., 2014, 6, 978–982 CrossRef CAS PubMed; (i) L. Wang, M. O. Vysotsky, A. Bogdan, M. Bolte and V. Böhmer, Science, 2004, 304, 1312–1314 CrossRef CAS PubMed.
- (a) S. Li, M. Liu, B. Zheng, K. Zhu, F. Wang, N. Li, X.-L. Zhao and F. Huang, Org. Lett., 2009, 11, 3350–3353 CrossRef CAS PubMed; (b) S. M. Goldup, D. A. Leigh, P. J. Lusby, R. T. McBurney and A. M. Z. Slawin, Angew. Chem., Int. Ed., 2008, 47, 6999–7003 CrossRef CAS PubMed; (c) S. Kang, B. M. Berkshire, Z. Xue, M. Gupta, C. Layode, P. A. May and M. F. Mayer, J. Am. Chem. Soc., 2008, 130, 15246–15247 CrossRef CAS PubMed; (d) A. F. M. Kilbinger, S. J. Cantrill, A. W. Waltman, M. W. Day and R. H. Grubbs, Angew. Chem., Int. Ed., 2003, 42, 3281–3285 CrossRef CAS PubMed; (e) R. G. E. Coumans, J. A. A. W. Elemans, P. Thordarson, R. J. M. Nolte and A. E. Rowan, Angew. Chem., Int. Ed., 2003, 42, 650–654 CrossRef CAS.
- (a) C. Dietrich-Buchecker, G. Rapenne and J.-P. Sauvage, Chem. Commun., 1997, 2053–2054 RSC; (b) J. Guo, P. C. Mayers, G. A. Breault and C. A. Hunter, Nat. Chem., 2010, 2, 218–222 CrossRef CAS PubMed; (c) J.-F. Ayme, G. Gil-Ramírez, D. A. Leigh, J.-F. Lemonnier, A. Markevicius, C. A. Muryn and G. Zhang, J. Am. Chem. Soc., 2014, 136, 13142–13145 CrossRef CAS PubMed; (d) C. Dietrich-Buchecker, G. Rapenne and J.-P. Sauvage, Coord. Chem. Rev., 1999, 185–186, 167–176 CrossRef CAS.
- M. R. Buchmeiser, Chem. Rev., 2000, 100, 1565–1604 CrossRef CAS PubMed.
- (a) N. M. Glagovich, E. M. Reed, G. Crundwell, J. B. Updegraff III, M. Zeller and A. D. Hunter, Acta Crystallogr., Sect. A: Found. Crystallogr., 2005, E61, o1251–o1253 Search PubMed; (b) R. Hadjikhani, S. Dehghanpour, A. Mahmoudi and F. Mojahed, Z. Anorg. Allg. Chem., 2006, 632, 723–725 CrossRef CAS; (c) T. W. Bell and F. Guzzo, J. Chem. Soc., Chem. Commun., 1986, 769–771 RSC; (d) O. Q. Munro, S. D. Joubert and C. D. Grimmer, Chem.–Eur. J., 2006, 12, 7987–7999 CrossRef CAS PubMed; (e) F. Franceschi, G. Guillemot, E. Solari, C. Floriani, N. Re, H. Birkedal and P. Pattison, Chem.–Eur. J., 2001, 7, 1468–1478 CrossRef CAS PubMed.
- (a) J. G. Smith and I. Ho, Tetrahedron Lett., 1971, 12, 3541–3544 CrossRef; (b) P. Li, I. J. Scowen, J. E. Davies and M. A. Halcrow, J. Chem. Soc., Dalton Trans., 1998, 3791–3800 RSC; (c) Y.-S. Lee, Y.-H. Cho, S.-J. Lee, J.-K. Bin, J.-H. Yang, G.-S. Chae and C.-H. Cheon, Tetrahedron, 2015, 71, 532–538 CrossRef CAS.
- Over 70 crystal structures in the CCDC†; for the parent compound, see: N. Bresciani-Pahor, M. Calligaris, P. Delise, G. Dodic, G. Nardin and L. Randaccio, J. Chem. Soc., Dalton Trans., 1976, 2478–2483 RSC.
- (a) J. Mahía, M. A. Maestro, M. Vázquez, M. R. Bermejo, A. M. González and M. Maneiro, Acta Crystallogr., Sect. A: Found. Crystallogr., 2000, C56, 492–493 Search PubMed; (b) P. G. Owston, R. Peters, E. Ramsammy, P. A. Tasker and J. Trotter, J. Chem. Soc., Chem. Commun., 1980, 1218–1220 RSC.
- E. Pidcock, Chem. Commun., 2005, 3457–3459 RSC.
- (a) T. Matsuura and H. Koshima, J. Photochem. Photobiol., C, 2005, 6, 7–24 CrossRef CAS; (b) M. Miyata, N. Tohnai, I. Hisaki and T. Sasaki, Symmetry, 2015, 7, 1914–1928 CrossRef.
- L. R. Dalton, L. P. Yu, M. Chen, L. S. Sapochak and C. Xu, Synth. Met., 1993, 54, 155–160 CrossRef CAS.
- (a) M. Brookhart, M. L. H. Green and G. Parkin, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 6908–6914 CrossRef CAS PubMed; (b) A. Ilie, C. I. Raţ, S. Scheutzow, C. Kiske, K. Lux, T. M. Klapötke, C. Silvestru and K. Karaghiosoff, Inorg. Chem., 2011, 50, 2675–2684 CrossRef CAS PubMed.
- N. L'Hermite, J.-F. Peyrat, P. Hildgen, J.-D. Brion and M. Alami, Synthesis, 2008, 1049–1060 Search PubMed.
- G. J. Kubas, Inorg. Synth., 1979, 19, 90–92 CrossRef CAS.
- P. Ionita, S. Afr. J. Chem., 2008, 61, 123–126 CAS.
- APEX2 v2014.9-0, Bruker AXS Inc., Madison, Wisconsin, USA, 2014 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra, CIF files for compounds 4, 5, 6 and 12. CCDC 1439669–1439672. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra25584h |
| This journal is © The Royal Society of Chemistry 2016 |