Multi-substituted triazatruxene-functionalized pyrene derivatives as efficient organic laser gain media†
Ming Sanga,
Sizhen Caoa,
Jianpeng Yia,
Jinjin Huanga,
Wen-Yong Lai*ab and
Wei Huang*ab
aKey Laboratory for Organic Electronics and Information Displays (KLOEID) & Institute of Advanced Materials (IAM), Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), Nanjing University of Posts & Telecommunications, 9 Wenyuan Road, Nanjing 210023, China. E-mail: iamwylai@njupt.edu.cn
bKey Laboratory of Flexible Electronics (KLOFE) & Institue of Advanced Materials (IAM), Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), Nanjing Tech University (NanjingTech), 30 South Puzhu Road, Nanjing 211816, China. E-mail: wei-huang@njtech.edu.cn
First published on 5th January 2016
Abstract
A set of pyrene derivatives substituted with multiple triazatruxene at the 1, 6- and 1, 3, 6, 8-positions, namely Py-2TAT and Py-4TAT, was synthesized and characterized. Their thermal, photophysical, and electrochemical properties were investigated in comparison with those of the pyrene and triazatruxene units to explore the relationship between the molecular architectures and corresponding properties. It is found that introducing a triazatruxene unit onto the rigid pyrene chromophore can effectively depress the crystalline nature of the pyrene and triazatruxene units, which endow the resulting materials with improved morphology properties, enhanced thermal stabilities, and favorable solution processiblity. Particularly, due to the integration of triazatruxene functional units, the resulting materials exhibit high-lying HOMO energy levels that are well matched with that of the PEDOT:PSS/ITO anode, leading to an improved hole-injection property. Solution-processed non-doped films exhibited prominent amplified spontaneous emission (ASE) and lasing characteristics. Low ASE thresholds of 150 nJ per pulse (1.4 kW cm−2) at 508 nm for Py-2TAT and 450 nJ per pulse (4.1 kW cm−2) at 530 nm for Py-4TAT were recorded. Consequently, one-dimensional (1D) distributed feed-back (DFB) lasers with low lasing threshold of 21 nJ per pulse (8.6 kW cm−2) was demonstrated. The selection of different combinations of grating periods and film thicknesses has provided the opportunity to fine tune the lasing wavelength of the DFB lasers in the range of 484–556 nm. The results indicated that construction of multi-substituted triazatruxene-pyrene architectures was beneficial to improving the electrical properties and thermal stabilities without largely sacrificing the optical properties, manifesting the potential of triazatruxene-functionalized pyrenes as efficient gain media for electrically pumped organic lasers.
Introduction
Great progress has been made, particularly in the past decade, in the search for organic semiconductor materials with attractive optoelectronic properties for applications in the next generation of optoelectronic devices.1,2 Recently, solution-processed organic emitters with desirable optical and electrical properties have attracted much attention for applications in organic light-emitting diodes (OLEDs)3–5 and organic lasers.6–8 To date, despite significant progress has been achieved in OLEDs, electrically pumped organic lasers have not been realized yet.9–14 In this context, the rational design and synthesis of novel organic emitters with excellent optical gains, high carrier mobility and enhanced thermal stability are highly demanded. Most of the existing organic emitters generally suffer from their intrinsic low carrier mobility despite the higher optical gains and lower threshold energy relative to their inorganic counterparts.8 Generally, high carrier mobility and high luminous efficiency are contradictory for organic emitters. Thus, a search for new efficient organic emitters as organic laser gain media with superior optical and electrical properties and a better understanding of the relationship between molecular structures and device properties are highly required.As far as we are concerned, high luminous efficiency is an important prerequisite to obtain high optical gains, which can be achieved by polycyclic aromatic units such as fluorene,15–17 anthracene18–20 and pyrene.21–23 Among these, pyrene has stood out as a promising building block because of its high photoluminescence quantum yields (PLQYs), high charge carrier mobility and excellent thermal stability.23 However, pyrene itself and its derivatives normally tend to aggregate, resulting in red-shifted emission and low PLQYs in solid states.21–23 The pyrene aggregation can be suppressed by introducing bulky groups and star-shaped structures.23 From a structural perspective, the facile chemical modification of the pyrene structure makes it possible to induce novel molecular architectures via introducing various substitutions, thus varying corresponding optoelectronic properties. For instance, Krotkus et al. developed a series of pyrenyl-disubstituted fluorene and carbazole compounds which demonstrated promising ASE performance with low ASE threshold (down to 20 kW cm−2) with pyrene derivatives dispersed in the inert polystyrene matrix at a rather large optimal (3–5 wt%) chromophore concentration.24 Although the electrical properties can be fine modulated via incorporating electroactive carbazole units onto pyrene cappers structures, the optimal performance was achieved in a blend system.
Triazatruxene (TAT) comprising three overlapping carbazole fragments has attracted increasing recent interests.25–27 High mobility has been recorded from triazatruxene derivatives.28 Ziessel et al. developed a series of monosubstituted triazatruxenes dyes which showed large fluorescence quantum yields and electrochemical properties as well as high hole mobility.29 Meanwhile, it is also reported that the thermal stability of the compounds can be greatly enhanced by incorporating triazatruxene in the molecular structure.30,31 In our previous work, we have developed a novel series of monodisperse star-shaped conjugated macromolecules based on truxene or triazatruxene core with oligofluorene arms, which exhibited promising electroluminescence, optical gain, and lasing performance.31–36
As a matter of fact, facile solution processibility of polymers is significant to reduce the concentration quenching in the device fabrication. In contrast to polymers, small molecules have demonstrated great advantages as they can possess well-defined structures and high purity. Combined with both advantages mentioned above, monodisperse conjugated oligomers have been proven to be the most potential system, which provide a valuable platform to clarify the relationship between structures and properties. Furthermore, these oligomers can be easily fabricated into the amorphous and homogeneous films, showing high morphological stability in the device manufacture. Therefore, multifunctional compounds based on pyrene moieties have been developed and studied for the state-of-art, solution-processed OLEDs. Recently, Qin et al. developed monodisperse pyrene derivatives containing four peripheral triphenylene dendrons and triphenylamine surface groups which can be used as blue emitters with high luminance and high operational stability.37
Inspired by the promising characteristics of pyrene and triazatruxene, we intended to introduce the two building blocks into a monodisperse star-shaped architecture with the aim to accomplish high electrical properties and thermal stabilities while maintaining favorable optical characteristics. Thus, a set of compounds which integrated the triazatruxene functional units onto the planar rigid pyrene chromophore at 1, 6- and 1, 3, 6, 8-positions (Py-2TAT and Py-4TAT) were designed and synthesized. Introduction of the alkyl chain onto the triazatruxene moiety enabled the resulting molecules to be solution processible. The thermal, photophysical, and electrochemical properties of Py-2TAT and Py-4TAT were investigated to explore the relationship between the molecular architectures and properties. The lasing wavelength of the distributed feed-back (DFB) lasers can be fine-tuned in the range of 484–556 nm via an appropriate choice of grating periods and film thicknesses. Consequently, for Py-2TAT, promising lasing performance with low ASE threshold of 150 nJ per pulse (6.8 μJ cm−2) and low lasing threshold of 21 nJ per pulse (8.6 kW cm−2) was demonstrated.
Results and discussion
Synthesis and characterization
Synthesis of triazatruxene-functionalized pyrene derivatives Py-nTAT is depicted in Scheme 1. 1,3,6,8-Tetrabromopyrene was synthesized according to the ref. 38. 1,6-Dibromopyrene was purchased from J&K Scientific Ltd. China. Triazatruxene (TAT) was prepared by reported methods39 and bromination of triazatruxene with N-bromosuccinimide (NBS) was synthesized according to the ref. 27. Pyrene derivatives substituted with triazatruxene at the 1,6-(Py-2TAT) and 1,3,6,8-positions (Py-4TAT) were efficiently obtained by consecutive two-step synthesis through boronic pinacol esters of monobrominated triazatruxene followed by mutiple Suzuki coupling reactions.36 The molecular structures and purities were adequately confirmed by 1H and 13C NMR spectroscopy, and MALDI-TOF mass spectrometry.Thermal and morphology properties
Thermal stabilities of triazatruxene-substituted pyrene derivatives were estimated using thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC), respectively. As shown in Fig. S1 and S2,† Py-2TAT and Py-4TAT exhibited good thermal stability with 5% weight loss decomposition temperature (Td) up to 378 °C and 376 °C, respectively. The glass transition temperature (Tg) for Py-4TAT is 89 °C, which is higher than Py-2TAT (57 °C). The thermal characteristics of the resulting molecules are summarized in Table 1. To identify the crystallinity of Py-nTAT materials, wide-angle X-ray diffraction (WXRD) was performed. From the WXRD patterns (Fig. 1), we clearly observe a transition from a polycrystalline to an amorphous state by increasing the triazatruxene substitutions from Py-2TAT to Py-4TAT. In contrast, sharp peaks for the pyrene core (Py) and triazatruxene unit (TAT) were observed. It is implied that integration the triazatruxene unit onto rigid pyrene chromophore can depress the crystalline nature of pyrene and triazatruxene units.| Compound | Tga, °C | Tdb, °C | λabs, nm (THF) | ε, [105 L mol−1 cm−1] (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) ε) |
λPL, nm (THF) | λabs, nm (film) | λPL, nm (film) | PLQYc |
|---|---|---|---|---|---|---|---|---|
| a Scan rate 10 °C min−1, N2 atmosphere.b Heating rate 10 °C min−1, N2 atmosphere.c Fluorescence quantum yields in solid state films, measured on quartz plates using an integrating sphere. | ||||||||
| Py-2TAT | 57 | 378 | 302, 399 | 0.9 (4.95), 0.29 (4.46) | 487 | 322, 413 | 487 | 0.16 |
| Py-4TAT | 89 | 376 | 304, 417 | 2.21 (5.34), 0.47 (4.67) | 493 | 322, 427 | 514 | 0.66 |
 | ||
| Fig. 1 WAXD patterns (5–50°) of TAT, Py and Py-nTAT powders. All samples were tested under the same conditions, and each pattern was demonstrated at its original intensity. | ||
Atomic force microscopy (AFM) images of the compounds were recorded on spectrosil substrates to investigate their morphological stability (Fig. 2). The thin films were prepared by spin-coating without any treatment. The thin film had a smooth surface morphology with a root-mean-square (RMS) roughness of 0.475 for Py-2TAT and 0.546 nm for Py-4TAT, respectively. For comparison, the films of TAT and Py were prepared, which showed rather coarse surface due to their high crystallinity. The trends are consistent with the XRD analysis. The results indicate that construction of multi-substituted triazatruxene-pyrene molecular architectures is helpful for improving the amorphous properties and film-forming properties that would largely facilitate the fabrication of much more homogeneous thin films by solution processing, which is desirable for organic optoelectronic devices.
Photophysical properties
The photophysical properties of TAT, Py and Py-nTAT were measured by UV-vis absorption and photoluminescence (PL) spectra in THF and in thin films (Fig. 3). TAT and Py show well-resolved absorption in dilute THF, whereas their absorption spectra in the thin film exhibit a strong π–π* transition with a broad tail at low energy, which might be related to excimers or aggregates of the rigid monomer molecules. In the film states, PL spectra of TAT and Py are relatively broader and red-shifted compared with those recorded in THF. Additionally, a weak shoulder at near 450 nm for TAT and a strong fluorescence band around 460 nm for Py can also be observed, which are attributed to the emission from aggregates formed by the rigid monomer molecules in the solid states.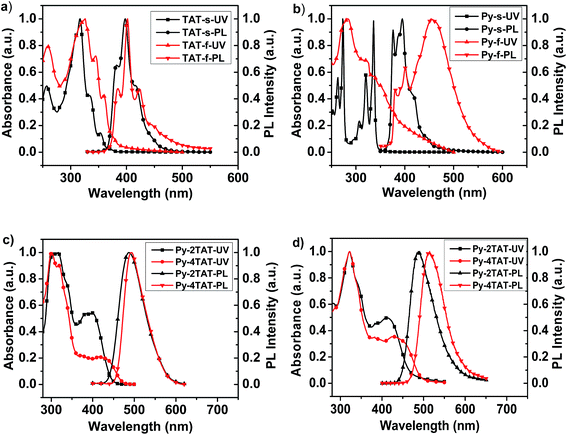 | ||
| Fig. 3 Normalized absorption and emission spectra of (a) TAT, (b) Py, and Py-nTAT in (c) THF and (d) film states. | ||
Py-nTAT exhibit prominent short and long wavelength absorption peaks (see Fig. 3c and d and Table 1). The shorter wavelength bands at 300–350 nm may originate from the triazatruxene moiety, while the longer bands at 350–480 nm are ascribed to the π–π* transition of the pyrene moiety. In THF solution, Py-4TAT shows red-shifted wavelength absorption maxima (λmax) at 304 nm and a shoulder peak at 417 nm in comparison to Py-2TAT (λ = 302 and 399 nm, respectively), owing to its slightly more extended conjugation lengths. In the film state, both Py-2TAT and Py-4TAT exhibit two characteristic absorption peaks, which are relatively red-shifted from those recorded for THF solutions. Both compounds show green emissions with the peaks at 487 and 493 nm for Py-2TAT and Py-4TAT, respectively. The film PL spectra for Py-2TAT are almost identical to those obtained in solution, while Py-4TAT displays emission peak at 514 nm, which are red-shifted about 21 nm relative to its solution spectra, indicating weak intermolecular interactions in thin films. Apparently, constructing multi-substituted architectures can effectively depress the π-stacking/aggregation tendency of the planar TAT units and the rigid Py core structure in the solid state. Photoluminescence quantum yields (PLQY) of these materials in the solid states measured on quartz plates using an integrating sphere are 0.16 for Py-2TAT and 0.66 for Py-4TAT. As a result, PLQY of tetrasubstituted pyrene compound Py-4TAT is much higher than that of disubstituted pyrene compound Py-2TAT, showing a comparable level to other tetrasubstituted pyrene derivatives but with more simple synthetic architectures.40,41
Fluorescence transients of TAT, Py and Py-nTAT were investigated to get further insight into the excited state relaxation processes. The transients measured at the fluorescence band maxima of the studied compounds in the dilute solutions and the neat films are presented in Fig. S3 and S4.† The emission decays of Py and TAT are recorded by employing two discrete exponentials in THF with excellent fits (Table S1†). In THF solution, single exponential decays are evident for both Py-2TAT and Py-4TAT with excited state lifetimes (τ) of 3.32 and 3.96 ns, respectively. The decay times are similar to those reported for 1-pyrene substituted derivatives24,42,43 and pyrene-cored dendrimers44,45 in the monomeric state.
The fluorescence transients of the neat films were fairly well described by using a double exponential decay model yielding two decay components with different fractional intensities, that is, different contributions to the overall decay profile, pointing out several different origins of the radiative transitions. In the films, Py-nTAT compounds possess two lifetime components, probably originating from their similar fluorescent backbones. The fast decay component (τ1) of the neat films is less than 1 ns (0.83 ns for Py-2TAT and 0.98 ns for Py-4TAT), which has a dominant contribution (fractional intensity 69–72%) in their fluorescence decay dynamics. The fast component may result from exciton migration, and/or the migration-induced exciton quenching.24 Bearing in mind the formation of molecular aggregates in the neat films, as evidenced from their fluorescence bands, the slow relaxation component with τ2 (2.38 ns for Py-2TAT and 2.55 ns for Py-4TAT) can be attributed to the aggregated states.24 In addition, Table S2† shows the average PL lifetimes (τ) for Py-2TAT and Py-4TAT are 1.30 and 1.42 ns, respectively. Reduced χ2 values, which are used together with weighted residuals as the goodness-of-fit criteria, do not exceed 1.2, ensuring a reliable description of the experimental data.
Electronic and electrochemical properties
The molecular structures of the Py-nTAT were optimized using density functional theory (DFT) method (B3LYP) with 6-31G (d) basis set by GAUSSIAN 09. For simplicity and clarity, the lengthy alkyl chains were replaced by methyl in the model structures. It was assumed that such a change would not affect the calculation results for relevant discussion here. The optimized geometries and calculated frontier orbitals of Py-2TAT and Py-4TAT are depicted in Fig. S5.† The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) values calculated with B3LYP/6-31G(d,p) level for Py-nTAT are summarized in Table 2.| Compound | Eoxb, (V) | Eredb, (V) | EcvHOMOc, (eV) | EcvLUMOc, (eV) | Eoptgd, (eV) | EcalHOMOe, (eV) | EcalLUMOe, (eV) | Ecalge, (eV) |
|---|---|---|---|---|---|---|---|---|
| a Cyclic voltammogram curves of drop-coated films measured in 0.1 M Bu4NPF6 acetonitrile solution at a scan rate of 50 mV s−1 at room temperature (versus Ag/Ag+).b Onset oxidation potentials versus Ag/Ag+.c Estimated from the onset oxidation and reduction potential by using EcvHOMO = −e(Eox + 4.71 V) and EcvLUMO = −e(Ered + 4.71 V).d Eoptg (optical gap) calculated from the onset of absorption used the formula: Eoptg = 1240/λonset.e HOMO and LUMO calculated with B3LYP/6-31G(d,p) level. | ||||||||
| Py-2TAT | 0.46 | −2.34 | −5.17 | −2.37 | 2.79 | −4.78 | −1.46 | 3.32 |
| Py-4TAT | 0.50 | −2.39 | −5.21 | −2.32 | 2.63 | −4.58 | −1.59 | 2.99 |
The optimized geometries show that the pyrene core and triazatruxene substitutions are significantly twisted, rendering the chair-shaped Py-2TAT molecule and bowl-shaped Py-4TAT molecule with a twisted nonplanar structure. The different numbers of triazatruxene attached to the pyrene core result in different molecular packing. Obviously, Py-4TAT causes more pronounced steric hindrance effects and, thus, is more favorable for preventing molecular aggregation in the solid state than Py-2TAT. Meanwhile, this assumption has been confirmed by thermal and morphology analysis as mentioned above. For Py-2TAT, the LUMO is dominated by the pyrene unit, while the HOMO is mainly delocalized on the whole molecule. However, for Py-4TAT, an obvious overlap exists between the HOMO and LUMO, indicating that delocalization occurs across over the pyrene core structure.
The electrochemical characteristics of Py-nTAT were studied by cyclic voltammetry (CV) to further reveal the electronic properties. The data are collected in Table 2, in comparison with those calculated with B3LYP/6-31G (d). As shown in Fig. 4, the onsets of the oxidation potentials were found to be 0.46 and 0.56 V for Py-2TAT and Py-4TAT, respectively. In Table 2, the HOMOs of Py-2TAT and Py-4TAT are calculated to be −5.17 and −5.21 eV, respectively. The LUMO levels are deduced to be −2.37 and −2.32 eV from the reduction potentials. Noticeably, it is found that integration of triazatruxene functional units onto the pyrene core endows the materials with HOMO levels that are well matched with that of the poly(3,4-ethylenedioxythiopene):poly (styrenesulfonate) (PEDOT:PSS)/ITO anode (−5.20 eV),46 leading to a significantly improved hole-injection property. Based on the red edge of the longest absorption wavelength for the solid-state sample, the optical band gaps (Eg) of Py-2TAT and Py-4TAT were estimated to be 2.79 and 2.63 eV, respectively. All the results indicate that DFT calculations follow similar trends as revealed in the CV study.
Amplified spontaneous emission
To reveal the optical gain properties, amplified spontaneous emission (ASE) properties of the triazatruxene-functionalized pyrene compounds Py-2TAT and Py-4TAT were examined without utilization of external resonators or patterned distributed feedback grating structures. As a simple and appropriate technique, ASE measurements are widely applied to compare the performance of different materials for lasing application excluding effects of resonant cavity.6,47When the sample was pumped at high excitation densities, we observed ASE from the edge of the Py-2TAT film, with a line-narrowed spectrum (Fig. 5) peaked at 508 nm, close to the 0–1 vibronic peak of the PL emission. This is consistent with a quasi-four-level vibronic system allowing low-threshold operation.7 Simultaneously, ASE from the edge of the Py-4TAT film was seen at 530 nm. Fig. 6 shows the full-width at half-maximum (FWHM) line width of the emission spectrum and relative output power as a function of the pump intensity. According to the results, the FWHM of Py-2TAT has dropped dramatically from 66 nm to 12 nm when pumped above a threshold intensity EASEth of 150 nJ (1.4 kW cm−2). As shown in the inset of Fig. 6a, the output emission is amplified when the energy of pumped laser pulse is above the threshold. In Fig. 6b, Py-4TAT has the same ASE characteristics as Py-2TAT, while the FWHM of the emission spectrum of Py-4TAT decreases to 8 nm with a line-narrowed spectrum peaked at 530 nm and a threshold intensity of 450 nJ (4.1 kW cm−2). Therefore, Py-2TAT has the lowest EASEth, which is comparable to other pyrene derivatives24 and much better than perylene derivatives (15–20 kW cm−2) under analogous conditions.48–50 It is worthwhile to note that non-doped organic lasers have great advantages over small molecules because they suffer little from the concentration quenching.
Organic lasing properties
One-dimensional (1D) distributed feedback (DFB) lasers were fabricated by spin-coating thin films of Py-nTAT on top of etched nano-printed 1D gratings (Fig. 7a). Their performances were tested under the same pulsed optical excitation as used for ASE studies. Above the laser threshold, the surface-emission spectrum has narrowed to a single peak of line width 0.1–0.3 nm line width. Typical laser spectra are shown in Fig. 7b for various lasers. By selecting different combinations of grating periods and film thicknesses, the lasing wavelength of the DFB lasers could be fine tuned in the range of 484–505 nm and 535–556 nm for Py-2TAT and Py-4TAT, respectively. For a grating with period of 290 nm, fill factor of 50%, and etch depth of 30 nm, low lasing threshold of 21 nJ per pulse (8.6 kW cm−2) was achieved for a laser based on Py-2TAT operating at 494 nm with a film thickness of 125 nm (Fig. 8a). For Py-4TAT (Fig. 8b), lasing threshold values of 60 nJ per pulse (24.5 kW cm−2) by using a grating with period of 350 nm, fill factor of 50%, and etch depth of 30 nm were recorded, which was 3-fold higher than that of Py-2TAT. Consequently, these lasing results match well with the ASE results and suggest that Py-2TAT exhibits superior ASE and lasing characteristics. The results suggest more bulky triazatruxene functional units integrated onto the pyrene core are detrimental to optical gain (Table 3). | ||
| Fig. 8 (a) Py-2TAT laser threshold of 21 nJ (8.6 kW cm−2) at the emission wavelength of 494 nm. (b) Py-4TAT laser threshold of 60 nJ (24.5 kW cm−2) at the emission wavelength of 547 nm. | ||
Py-nTAT DFB lasers with different grating periods. For Py-2TAT, lasing from 484 (1), 494 (2) and 505 nm (3) are from 280, 290 and 300 nm period 1D grating, respectively. For Py-4TAT, lasing from 535 (4), 547 (5) and 556 nm (6) are from 340, 350 and 360 nm period 1D grating, respectively. Film thicknesses: 125 nm for Py-2TAT and 100 nm for Py-4TAT.
Conclusions
In summary, we have designed and synthesized a set of new pyrene derivatives substituted with triazatruxene at 1,6- and 1, 3, 6, 8-positions. The combination of pyrene and triazatruxene units into a monodisperse multi-substituted molecular architecture results in improved thermal stabilities, facile solution processability, and enhanced electrical properties while maintaining promising optical gain properties. Specifically, integration of triazatruxene functional units onto the pyrene core endows the materials with high-lying HOMO energy levels that are well matched with that of the PEDOT:PSS/ITO anode (−5.20 eV), leading to an improved hole-injection property. Non-doped organic lasers based on these materials by solution processing exhibit highly efficient luminescence and promising lasing characteristics. Meanwhile, the selection of different combinations of grating periods and film thicknesses has provided the opportunity to fine tune the lasing wavelength of the DFB lasers in the range of 484–556 nm. In this way, low ASE threshold of 150 nJ per pulse (1.4 kW cm−2) and low lasing threshold of 21 nJ per pulse (8.6 kW cm−2) were demonstrated for triazatruxene-disubstituted pyrene derivatives. The results indicated that such a design strategy was beneficial to improving the electrical properties and thermal stabilities without largely sacrificing the optical properties, manifesting the great potential of triazatruxene-functionalized pyrenes as efficient gain media for electrically pumped organic lasers.Experimental section
Materials
All the reagents used were purchased from Sigma-Aldrich, J&K or Xiya Reagent (China). When necessary, solvents and reagents were purified using standard procedures.Characterization
NMR spectra were recorded on a Bruker Ultra Shield Plus 400 MHz NMR (1H: 400 MHz, 13C: 100 MHz). The matrix assisted laser desorption ionization time of flight mass spectroscopy (MALDI-TOF MS) measurements were carried out with a Shimadzu AXIMA-CFR mass spectrometer. UV-visible absorption spectra were recorded on a Shimadzu UV-3600 UV-vis-NIR spectrophotometer. Photoluminescence (PL) spectra were measured using a RF-5301 PC spectrofluorophotometer. Lifetimes were measured using an Edinburgh FLSP920 lifetime spectrometer with a 375 nm laser (typical pulse width: 55 ps; pulse repetition frequencies: 20 MHz); the fluorescence quantum yields of films were measured with an integration sphere. Electrochemical behavior was investigated by cyclic voltammetry (CV) with a standard three-electrode electrochemical cell in a 0.1 M tetra-n-butylammonium hexafluorophosphate (Bu4NPF6) in nonaqueous acetonitrile at room temperature under nitrogen with a scanning rate of 50 mV s−1. A platinum working electrode, a glassy carbon electrode, and an Ag/AgNO3 (0.1 M) reference electrode were used. The CV curves were calibrated using ferrocene/ferrocenium (Fc/Fc+) redox couple (4.8 eV below the vacuum level) as the internal standard. The formal potential of Fc/Fc+ was measured as 0.09 V against Ag/Ag+. Thus, the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) energy levels could be calculated according to: EHOMO = −e(Eox + 4.71 V), ELUMO = −e(Ered + 4.71 V); where Eox and Ered is the onset oxidation and reduction potential, respectively. Differential scanning calorimetry (DSC) and thermo-gravimetric analysis (TGA) were done on Shimadzu DSC-60A and DTG-60A equipment, respectively. The surface morphology of the films was investigated by atomic force microscopy (AFM, Bruker Dimension Icon). The molecular structures of the Py-nTAT were optimized using density functional theory (DFT) method (B3LYP) with 6-31G (d) basis set by GAUSSIAN 09.Device fabrication and measurements
Thin-film samples (100–225 nm thickness) for optical measurements (absorption and PL spectra, PLQY, PL decay, and ASE measurements) were prepared by spin-coating starburst solutions (comprising 35 mg mL−1 of Py-2TAT or 20 mg mL−1 of Py-4TAT in CHCl3) onto precleaned spectrosil substrates. DFB lasers were fabricated by nanoimprint lithography (NIL) method and then Py-2TAT and Py-4TAT were spin-coated on top.For ASE measurements, the samples were optically pumped with the pulsed (5 ns, 10 Hz, 355 nm) output of a Q-switched Nd:YAG laser pumped optical parametric amplifier (Spectron SL 450) focused with a cylindrical lens to form a 4 mm × 0.55 mm stripe-shaped excitation area on the sample. For DFB lasers, the pump excitation area employed for monitoring laser emission was a circular spot with 250 μm in diameter (4.9 × 10−4 cm2 in area). The pulse energy incident on the sample was adjusted by the insertion of calibrated neutral density filters into the beam path. The laser emission was monitored with a fiber-coupled spectrograph and a charge coupled device (CCD) detector. The pump laser beam was incident on the sample at an angle of 45° to the surface normal in order to allow spatial separation at the CCD detector of the DFB laser output (emitted normal to the film plane) and reflected light from the strong pump.
Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to get 2 as a white solid (0.35 g, 60%). MALDI-TOF MS (m/z): calcd for C42H50BrN3, exact mass: 675.32, mol. wt.: 676.77; found: 675.46. 1H NMR (400 MHz, CDCl3): δ 8.25 (dd, 2H, J = 14.8, 8.0 Hz), 8.06 (d, 1H, J = 8.6 Hz), 7.72 (s, 1H), 7.62 (t, 2H, J = 7.1 Hz), 7.52–7.38 (m, 3H), 7.35 (t, 2H, J = 6.4 Hz), 4.93–4.74 (m, 6H), 1.96 (s, 6H), 1.47–1.09 (m, 18H), 0.82 (s, 9H). 13C NMR (100 MHz, CDCl3): δ 141.9, 141.0, 139.0, 138.4, 123.3, 122.4, 121.6, 119.7, 116.0, 113.3, 110.9, 103.1, 47.1, 31.4, 29.7, 26.2, 22.7, 14.1.
1) to get 2 as a white solid (0.35 g, 60%). MALDI-TOF MS (m/z): calcd for C42H50BrN3, exact mass: 675.32, mol. wt.: 676.77; found: 675.46. 1H NMR (400 MHz, CDCl3): δ 8.25 (dd, 2H, J = 14.8, 8.0 Hz), 8.06 (d, 1H, J = 8.6 Hz), 7.72 (s, 1H), 7.62 (t, 2H, J = 7.1 Hz), 7.52–7.38 (m, 3H), 7.35 (t, 2H, J = 6.4 Hz), 4.93–4.74 (m, 6H), 1.96 (s, 6H), 1.47–1.09 (m, 18H), 0.82 (s, 9H). 13C NMR (100 MHz, CDCl3): δ 141.9, 141.0, 139.0, 138.4, 123.3, 122.4, 121.6, 119.7, 116.0, 113.3, 110.9, 103.1, 47.1, 31.4, 29.7, 26.2, 22.7, 14.1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to produce compound 3 as a pale yellow oil (0.54 g, 51%). MALDI-TOF MS (m/z): calcd for C48H62BN3O2, exact mass: 723.49, mol. wt.: 723.84; found: 723.84. 1H NMR (400 MHz, CDCl3): δ 8.30 (d, 3H, J = 7.9 Hz), 8.11 (s, 1H), 7.81 (d, 1H, J = 8.0 Hz), 7.64 (d, 2H, J = 8.0 Hz), 7.46 (t, 2H, J = 7.5 Hz), 7.36 (d, 2H, J = 4.1 Hz), 5.03–4.96 (m, 6H), 4.96–4.87 (m, 6H), 2.13–1.89 (m, 12H), 1.46 (s, 18H), 0.95–0.74 (m, 9H). 13C NMR (100 MHz, CDCl3): δ 141.0, 140.9, 140.5, 139.6, 139.3, 138.9, 125.9, 123.5, 123.4, 122.7, 121.6, 121.4, 120.6, 119.7, 119.6, 116.9, 110.5, 110.4, 103.2, 83.7, 77.3, 77.0, 76.7, 47.1, 47.0, 31.5, 31.4, 31.3, 29.7, 26.4, 26.3, 26.2, 25.0, 22.5, 22.4, 13.9.
1) to produce compound 3 as a pale yellow oil (0.54 g, 51%). MALDI-TOF MS (m/z): calcd for C48H62BN3O2, exact mass: 723.49, mol. wt.: 723.84; found: 723.84. 1H NMR (400 MHz, CDCl3): δ 8.30 (d, 3H, J = 7.9 Hz), 8.11 (s, 1H), 7.81 (d, 1H, J = 8.0 Hz), 7.64 (d, 2H, J = 8.0 Hz), 7.46 (t, 2H, J = 7.5 Hz), 7.36 (d, 2H, J = 4.1 Hz), 5.03–4.96 (m, 6H), 4.96–4.87 (m, 6H), 2.13–1.89 (m, 12H), 1.46 (s, 18H), 0.95–0.74 (m, 9H). 13C NMR (100 MHz, CDCl3): δ 141.0, 140.9, 140.5, 139.6, 139.3, 138.9, 125.9, 123.5, 123.4, 122.7, 121.6, 121.4, 120.6, 119.7, 119.6, 116.9, 110.5, 110.4, 103.2, 83.7, 77.3, 77.0, 76.7, 47.1, 47.0, 31.5, 31.4, 31.3, 29.7, 26.4, 26.3, 26.2, 25.0, 22.5, 22.4, 13.9.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford Py-2TAT as a yellow-green solid (0.17 g, 60%). MALDI-TOF MS (m/z): calcd for C100H108N6, exact mass: 1392.86, mol. wt.: 1393.97; found: 1394.28. 1H NMR (400 MHz, CDCl3): δ 8.47 (d, J = 9.4 Hz, 4H), 8.33 (t, J = 7.9 Hz, 6H), 8.24 (d, J = 7.8 Hz, 2H), 8.15 (d, J = 9.4 Hz, 2H), 7.92 (s, 2H), 7.68 (t, J = 7.7 Hz, 6H), 7.49 (dd, J = 14.4, 7.1 Hz, 4H), 7.37 (dd, J = 14.2, 7.0 Hz, 4H), 5.08–4.93 (m, 12H), 2.16–1.99 (m, 12H), 1.35–1.21 (m, 36H), 0.87–0.80 (m, 18H). 13C NMR (100 MHz, CDCl3): δ 141.2, 141.1, 141.0, 139.4, 139.0, 138.8, 138.7, 135.8, 130.4, 129.3, 127.3, 125.5, 124.8, 124.4, 123.8, 123.5, 123.4, 123.0, 122.7, 121.5, 121.2, 119.7, 112.7, 110.5, 103.3, 103.1, 77.3, 77.2, 77.0, 76.7, 47.1, 31.5, 31.4, 29.9, 29.8, 26.4, 26.3, 22.5, 22.4, 13.9.
1) to afford Py-2TAT as a yellow-green solid (0.17 g, 60%). MALDI-TOF MS (m/z): calcd for C100H108N6, exact mass: 1392.86, mol. wt.: 1393.97; found: 1394.28. 1H NMR (400 MHz, CDCl3): δ 8.47 (d, J = 9.4 Hz, 4H), 8.33 (t, J = 7.9 Hz, 6H), 8.24 (d, J = 7.8 Hz, 2H), 8.15 (d, J = 9.4 Hz, 2H), 7.92 (s, 2H), 7.68 (t, J = 7.7 Hz, 6H), 7.49 (dd, J = 14.4, 7.1 Hz, 4H), 7.37 (dd, J = 14.2, 7.0 Hz, 4H), 5.08–4.93 (m, 12H), 2.16–1.99 (m, 12H), 1.35–1.21 (m, 36H), 0.87–0.80 (m, 18H). 13C NMR (100 MHz, CDCl3): δ 141.2, 141.1, 141.0, 139.4, 139.0, 138.8, 138.7, 135.8, 130.4, 129.3, 127.3, 125.5, 124.8, 124.4, 123.8, 123.5, 123.4, 123.0, 122.7, 121.5, 121.2, 119.7, 112.7, 110.5, 103.3, 103.1, 77.3, 77.2, 77.0, 76.7, 47.1, 31.5, 31.4, 29.9, 29.8, 26.4, 26.3, 22.5, 22.4, 13.9.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford Py-4TAT as a yellow solid (0.26 g, 54%). MALDI-TOF MS (m/z): calcd for C184H206N12, exact mass: 2583.65, mol. wt.: 2585.68; found: 2583.92. 1H NMR (400 MHz, CDCl3): δ 8.59 (s, 4H), 8.56–8.46 (m, 6H), 8.32 (d, J = 7.9 Hz, 8H), 8.07 (s, 4H), 7.82 (d, J = 8.2 Hz, 4H), 7.66 (t, J = 7.7 Hz, 8H), 7.47 (dd, J = 15.1, 7.5 Hz, 8H), 7.36 (dd, J = 16.3, 8.2 Hz, 8H), 4.97 (dd, J = 22.9, 15.4 Hz, 24H), 2.16–1.97 (m, 24H), 1.25 (ddd, J = 29.4, 27.4, 17.1 Hz, 72H), 0.96–0.69 (m, 36H). 13C NMR (100 MHz, CDCl3): δ 141.3, 141.0, 139.4, 139.0, 138.8, 135.8, 130.5, 128.6, 126.5, 125.7, 123.5, 123.4, 122.8, 122.7, 121.5, 121.2, 119.7, 112.7, 110.5, 103.3, 103.1, 47.1, 34.7, 33.1, 31.5, 31.4, 29.9, 29.8, 26.9, 26.4, 26.3, 22.5, 22.4, 13.9, 13.8.
1) to afford Py-4TAT as a yellow solid (0.26 g, 54%). MALDI-TOF MS (m/z): calcd for C184H206N12, exact mass: 2583.65, mol. wt.: 2585.68; found: 2583.92. 1H NMR (400 MHz, CDCl3): δ 8.59 (s, 4H), 8.56–8.46 (m, 6H), 8.32 (d, J = 7.9 Hz, 8H), 8.07 (s, 4H), 7.82 (d, J = 8.2 Hz, 4H), 7.66 (t, J = 7.7 Hz, 8H), 7.47 (dd, J = 15.1, 7.5 Hz, 8H), 7.36 (dd, J = 16.3, 8.2 Hz, 8H), 4.97 (dd, J = 22.9, 15.4 Hz, 24H), 2.16–1.97 (m, 24H), 1.25 (ddd, J = 29.4, 27.4, 17.1 Hz, 72H), 0.96–0.69 (m, 36H). 13C NMR (100 MHz, CDCl3): δ 141.3, 141.0, 139.4, 139.0, 138.8, 135.8, 130.5, 128.6, 126.5, 125.7, 123.5, 123.4, 122.8, 122.7, 121.5, 121.2, 119.7, 112.7, 110.5, 103.3, 103.1, 47.1, 34.7, 33.1, 31.5, 31.4, 29.9, 29.8, 26.9, 26.4, 26.3, 22.5, 22.4, 13.9, 13.8.Acknowledgements
The authors acknowledge financial support from the National Key Basic Research Program of China (973 Program, 2014CB648300), the National Natural Science Foundation of China (21422402, 20904024, 51173081, 61136003), the Natural Science Foundation of Jiangsu Province (BK20140060, BK20130037, BM2012010), Program for Jiangsu Specially – Appointed Professors (RK030STP15001), Program for New Century Excellent Talents in University (NCET-13-0872), Specialized Research Fund for the Doctoral Program of Higher Education (20133223110008, and 20113223110005), the Ministry of Education of China (IRT1148), the Synergetic Innovation Center for Organic Electronics and Information Displays, the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the NUPT “1311 Project”, the Six Talent Plan (2012XCL035), the 333 Project (BRA2015374) and the Qing Lan Project of Jiangsu Province.References
- Y. Shirota and H. Kageyama, Chem. Rev., 2007, 107, 953–1010 CrossRef CAS PubMed.
- J. Wu, W. Pisula and K. Müllen, Chem. Rev., 2007, 107, 718–747 CrossRef CAS PubMed.
- P. Strohriegl and J. V. Grazulevicius, Adv. Mater., 2002, 14, 1439–1452 CrossRef CAS.
- P. L. Burn, S. C. Lo and I. D. W. Samuel, Adv. Mater., 2007, 19, 1675–1688 CrossRef CAS.
- A. L. Kanibolotsky, I. F. Perepichkaz and P. J. Skabara, Chem. Soc. Rev., 2010, 39, 2695–2728 RSC.
- I. D. W. Samuel and G. A. Turnbull, Chem. Rev., 2007, 107, 1272–1295 CrossRef CAS PubMed.
- S. Chénais and S. Forget, Polym. Int., 2012, 61, 390–406 CrossRef.
- S. Z. Bisri, T. Takenobu and Y. Iwasa, J. Mater. Chem. C, 2014, 2, 2827 RSC.
- B. K. Yap, R. Xia, M. Campoy-Quiles, P. N. Stavrinou and D. D. Bradley, Nat. Mater., 2008, 7, 376–380 CrossRef CAS PubMed.
- D. Amarasinghe, A. Ruseckas, A. E. Vasdekis, G. A. Turnbull and I. D. Samuel, Adv. Mater., 2009, 21, 107–110 CrossRef CAS.
- E. B. Namdas, M. Tong, P. Ledochowitsch, S. R. Mednick, J. D. Yuen, D. Moses and A. J. Heeger, Adv. Mater., 2009, 21, 799–802 CrossRef CAS.
- H. Kim, N. Schulte, G. Zhou, K. Müllen and F. Laquai, Adv. Mater., 2011, 23, 894–897 CrossRef CAS PubMed.
- X. F. Jiang, Y. F. Xiao, C. L. Zou, L. He, C. H. Dong, B. B. Li, Y. Li, F. W. Sun, L. Yang and Q. Gong, Adv. Mater., 2012, 24, 260–264 CrossRef.
- M. M. Mróz, G. Sforazzini, Y. Zhong, K. S. Wong, H. L. Anderson, G. Lanzani and J. Cabanillas-Gonzalez, Adv. Mater., 2013, 25, 4347–4351 CrossRef PubMed.
- B. Liu, W.-L. Yu, Y.-H. Lai and W. Huang, Macromolecules, 2000, 33, 8945–8952 CrossRef CAS.
- B. Liu, W.-L. Yu, Y.-H. Lai and W. Huang, Chem. Mater., 2001, 13, 1984–1991 CrossRef CAS.
- G. Zeng, W.-L. Yu, S.-J. Chua and W. Huang, Macromolecules, 2002, 35, 6907–6914 CrossRef CAS.
- C. W. Tang and S. VanSlyke, Appl. Phys. Lett., 1987, 51, 913–915 CrossRef CAS.
- J. Shi and C. W. Tang, Appl. Phys. Lett., 2002, 80, 3201–3203 CrossRef CAS.
- Y. H. Kim, H. C. Jeong, S. H. Kim, K. Yang and S. K. Kwon, Adv. Funct. Mater., 2005, 15, 1799–1805 CrossRef CAS.
- C. Tang, F. Liu, Y.-J. Xia, J. Lin, L.-H. Xie, G.-Y. Zhong, Q.-L. Fan and W. Huang, Org. Electron., 2006, 7, 155–162 CrossRef CAS.
- C. Tang, F. Liu, Y.-J. Xia, L.-H. Xie, A. Wei, S.-B. Li, Q.-L. Fan and W. Huang, J. Mater. Chem., 2006, 16, 4074–4080 RSC.
- T. M. Figueira-Duarte and K. Müllen, Chem. Rev., 2011, 111, 7260–7314 CrossRef CAS PubMed.
- S. Krotkus, K. Kazlauskas, A. Miasojedovas, A. Gruodis, A. Tomkeviciene, J. V. Grazulevicius and S. Jursenas, J. Phys. Chem. C, 2012, 116, 7561–7572 CAS.
- L. Ji, Q. Fang, M. S. Yuan, Z. Q. Liu, Y. X. Shen and H. F. Chen, Org. Lett., 2010, 12, 5192–5195 CrossRef CAS PubMed.
- T. Bura, N. Leclerc, R. Bechara, P. Lévêque, T. Heiser and R. Ziessel, Adv. Energy Mater., 2013, 3, 1118–1124 CrossRef CAS.
- C. Ruiz, J. T. López Navarrete, M. C. Ruiz Delgado and B. Gómez-Lor, Org. Lett., 2015, 17, 2258 CrossRef CAS PubMed.
- A. Benito-Hernández, U. K. Pandey, E. Cavero, R. Termine, E. M. García-Frutos, J. L. Serrano, A. Golemme and B. Gómez-Lor, Chem. Mater., 2013, 25, 117–121 CrossRef.
- T. Bura, N. Leclerc, S. Fall, P. Lévêque, T. Heiser and R. Ziessel, Org. Lett., 2011, 13, 6030–6033 CrossRef CAS PubMed.
- J. Shao, Z. Guan, Y. Yan, C. Jiao, Q.-H. Xu and C. Chi, J. Org. Chem., 2011, 76, 780–790 CrossRef CAS PubMed.
- W. Y. Lai, Q. Y. He, R. Zhu, Q. Q. Chen and W. Huang, Adv. Funct. Mater., 2008, 18, 265–276 CrossRef CAS.
- W. Y. Lai, Q. Q. Chen, Q. Y. He, Q. L. Fan and W. Huang, Chem. Commun., 2006, 1959–1961 RSC.
- W. Y. Lai, R. Zhu, Q. L. Fan, L. T. Hou, Y. Cao and W. Huang, Macromolecules, 2006, 39, 3707–3709 CrossRef CAS.
- R. Zhu, W.-Y. Lai, H.-Y. Wang, N. Yu, W. Wei, B. Peng, W. Huang, L.-T. Hou, J.-B. Peng and Y. Cao, Appl. Phys. Lett., 2007, 90, 141909 CrossRef.
- W. Y. Lai, R. Xia, Q. Y. He, P. A. Levermore, W. Huang and D. D. Bradley, Adv. Mater., 2009, 21, 355–360 CrossRef CAS.
- W. Y. Lai, R. Xia, D. D. Bradley and W. Huang, Chem.–Eur. J., 2010, 16, 8471–8479 CrossRef CAS PubMed.
- T. Qin, W. Wiedemair, S. Nau, R. Trattnig, S. Sax, S. Winkler, A. Vollmer, N. Koch, M. Baumgarten, E. J. List and K. Mullen, J. Am. Chem. Soc., 2011, 133, 1301–1303 CrossRef CAS PubMed.
- V. de Halleux, J. P. Calbert, P. Brocorens, J. Cornil, J. P. Declercq, J. L. Brédas and Y. Geerts, Adv. Funct. Mater., 2004, 14, 649–659 CrossRef CAS.
- M. Franceschin, L. Ginnari-Satriani, A. Alvino, G. Ortaggi and A. Bianco, Eur. J. Org. Chem., 2010, 2010, 134–141 CrossRef.
- R. Muangpaisal, M.-C. Ho, T.-H. Huang, C.-H. Chen, J.-Y. Shen, J.-S. Ni, J. T. Lin, T.-H. Ke, L.-Y. Chen, C.-C. Wu and C. Tsai, Org. Electron., 2014, 15, 2148–2157 CrossRef CAS.
- G. Zhang, M. Baumgarten, M. Auer, R. Trattnig, E. J. List-Kratochvil and K. Mullen, Macromol. Rapid Commun., 2014, 35, 1931–1936 CrossRef CAS PubMed.
- R. D. Xia, W. Y. Lai, P. A. Levermore, W. Huang and D. D. C. Bradley, Adv. Funct. Mater., 2009, 19, 2844–2850 CrossRef CAS.
- A. G. Crawford, A. D. Dwyer, Z. Liu, A. Steffen, A. Beeby, L.-O. Pålsson, D. J. Tozer and T. B. Marder, J. Am. Chem. Soc., 2011, 133, 13349–13362 CrossRef CAS PubMed.
- T. Oyamada, S. Akiyama, M. Yahiro, M. Saigou, M. Shiro, H. Sasabe and C. Adachi, Chem. Phys. Lett., 2006, 421, 295–299 CrossRef CAS.
- M. Gingras, V. Placide, J. M. Raimundo, G. Bergamini, P. Ceroni and V. Balzani, Chem.–Eur. J., 2008, 14, 10357–10363 CrossRef CAS PubMed.
- T. Brown, J. Kim, R. Friend, F. Cacialli, R. Daik and W. Feast, Appl. Phys. Lett., 1999, 75, 1679–1681 CrossRef CAS.
- N. Tessler, Adv. Mater., 1999, 11, 363–370 CrossRef CAS.
- E. M. Calzado, P. G. Boj and M. A. Díaz-García, Int. J. Mol. Sci., 2010, 11, 2546–2565 CrossRef CAS PubMed.
- L. Cerdán, A. Costela, G. Durán-Sampedro, I. García-Moreno, M. Calle, M. Juan-y-Seva, J. de Abajo and G. A. Turnbull, J. Mater. Chem., 2012, 22, 8938–8947 RSC.
- A. Miasojedovas, K. Kazlauskas, G. Armonaite, V. Sivamurugan, S. Valiyaveettil, J. Grazulevicius and S. Jursenas, Dyes Pigm., 2012, 92, 1285–1291 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra25530a |
| This journal is © The Royal Society of Chemistry 2016 |






