Palladium-catalyzed C-3 desulfitative arylation of indolizines with sodium arylsulfinates and arylsulfonyl hydrazides†
Abstract
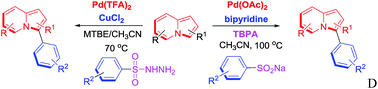
Derivatized indolizines were efficiently prepared by direct C-3 arylation of indolizines using sodium arylsulfinates and arylsulfonyl hydrazides. Pd-catalyzed desulfitative C-3 arylation with sodium arylsulfinates was achieved with the assistance of peroxides, and the catalytic efficiency was promoted by N-containing ligands. Arylsulfonyl hydrazides were also successfully applied in Pd-catalyzed desulfitative C-3 arylation with indolizines, and the side homocoupling reactions can be restrained in the component solvent under milder conditions. Various derivatives were synthesized in good yields by both methods, offering expedient protocols for the synthesis of C-3 functionalized indolizine molecules.
- This article is part of the themed collection: Editors’ collection: Catalytic Organic Transformations

 Please wait while we load your content...
Please wait while we load your content...