In situ synthesis of carbon nanotube doped metal–organic frameworks for CO2 capture†
Abstract
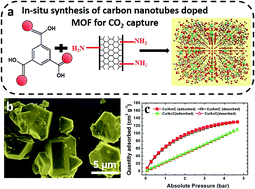
Metal organic–frameworks (MOFs) with intriguing structural motifs and unique properties are potential candidates for carbon dioxide (CO2) storage. Although structures with the single functional constructions and micropores were demonstrated to capture CO2 with high capacities at low temperature, their feeble interactions still limit practical applications at room temperature. Herein, we report in situ growth observation of hierarchical pores in copper(II) benzene-1,3,5-tricarboxylate (Cu-BTC) doped MOFs which gives high adsorption and enhances the CO2 binding ability. Thus, understanding this CO2-capturing mechanism, which has been causing controversy, is crucial for further development toward advanced study. The doped MOFs exhibit high specific surface areas of 1180 m2 g−1 and show good capacity to store CO2, which is mainly due to the presence of acid and amine functionalized CNTs and a large amount of narrow micropores (<1.0 nm).

 Please wait while we load your content...
Please wait while we load your content...