Efficient pyrene-imidazole derivatives for organic light-emitting diodes†
Abstract
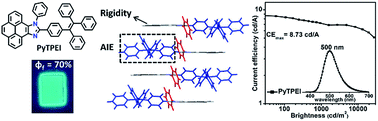
Two light emitting materials, 9-phenyl-10-(4-(1,2,2-triphenylvinyl)phenyl)-9H-pyreno[4,5-d]imidazole (PyTPEI) and 9-phenyl-10-(4′-(1,2,2-triphenylvinyl)-[1,1′-biphenyl]-4-yl)-9H-pyreno[4,5-d]imidazolepyreneimidazole (PyPTPEI), containing pyrene, imidazole and tetraphenylethene units are synthesized with high yields. Both of them exhibit good thermal stability with decomposition temperatures of 458 °C and 474 °C, respectively. The fluorescent quantum efficiency in amorphous films are as high as 70% for PyTPEI and 63% for PyPTPEI, respectively. In particular, PyPTPEI shows blue-shifted emission due to the more twisted conformation and reduced intermolecular interactions as compared with PyTPEI. The maximum current efficiency and maximum brightness of a non-doped OLED device using PyTPEI as an active layer reaches 8.73 cd A−1 and 27 419 cd m−2, which is better than that of PyPTPEI (7.68 cd A−1 and 19 419 cd m−2).

 Please wait while we load your content...
Please wait while we load your content...