Synergistic inhibition of carbon steel corrosion in 0.5 M HCl solution by indigo carmine and some cationic organic compounds: experimental and theoretical studies
Abstract
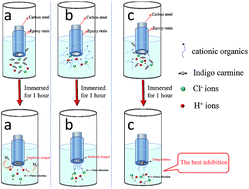
The synergistic corrosion inhibition effect of indigo carmine and three kinds of cationic organic compounds on 1045 carbon steel (CS) in 0.5 M HCl solution is reported. Electrochemical measurements showed that these three cationic organic compounds combined with indigo carmine reduce the speed of corrosion on 1045 CS and act as effective inhibitors. The combination of indigo carmine with BAB resulted in the best synergistic corrosion inhibition effect (S = 17.14), and the best inhibition efficiency (95.0%). SEM images and XPS data of the corroded steel surfaces suggested that the indigo disulphonate anion and organic cation could be simultaneously adsorbed on the CS surface to inhibit the corrosion of iron. The synergistic inhibition mechanism was investigated by dynamic simulations using quantum chemistry.

 Please wait while we load your content...
Please wait while we load your content...