Synthesis of novel fluorescent 12a-aryl substituted indoxylisoquinolines via aryne-induced domino process†
A. V. Varlamova,
N. I. Guranovaa,
R. A. Novikovb,
V. V. Ilyushenkovaa,
V. N. Khrustaleva,
N. S. Baleevac,
T. N. Borisovaa and
L. G. Voskressensky*a
aOrganic Chemistry Department, Peoples' Friendship University of Russia, 6, Miklukho-Maklaya St., Moscow 117198, Russia. E-mail: lvoskressensky@sci.pfu.edu.ru
bN. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, 47, Leninskij avenue, Moscow 119991, Russia
cInstitute of Bioorganic Chemistry, Russian Academy of Sciences, 16/10, Miklukho-Maklaya St, Moscow 117997, Russia
First published on 22nd January 2016
Abstract
A novel effective protocol towards indolo[2,1-a]isoquinolinones via aryne-induced migration of aryl-anion in aryloxy substituted 3,4-dihydroisoquinolines is reported. These novel 12a-aryl substituted indoxylisoquinolines exhibit fluorescence properties and are characterized by green-shifted spectra and moderate quantum yields.
Introduction
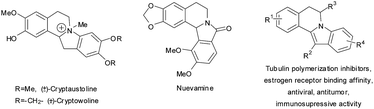
Reactive intermediates such as radicals, carbenes, nitrenes etc. have become widely used in organic synthesis. In recent decades arynes, highly reactive intermediates, justly converted from an object of purely theoretical interest into a reagent for synthesizing variously constructed N- and O-containing heterocycles1 after the development of the synthetic method of Kobayashi et al.2 Although [2 + 4] cycloaddition3 and 1,3-dipolar cycloaddition4 of arynes are used most frequently for heterocycle synthesis, the nucleophilic addition to arynes is one of the oldest and still widely used approaches towards alkaloids and biologically active compounds.5 The indolo[2,1-a]isoquinolines are the principal structural moieties of Cryptaustoline and Cryptowoline alkaloids,6 which along with their synthetic analogs exhibit strong anticancer activity and affinity for estrogen receptors.7 The structure of indoxylisoquinoline is also found in Nuevamine alkaloid (Fig. 1).8 However the methods for the construction of such a heterocycle core are limited.9The low-lying LUMO determines the highly electrophilic nature of arynes and makes them react primarily with electron-rich atoms of soft and neutral nucleophiles with the formation of zwitterions.10 If the substrate has an electrophilic site, especially carbonyl or imine groups, then the anionic center of the zwitterion can attack it furnishing a fused heterocyclic system.3f,4g,5f,10h,i,11
We presumed that the interaction of dehydrobenzene and derivatives thereof with the conjugated –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C
C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O system in aryloxy substituted isoquinolines would lead to annulation of an indole moiety and construction of the attractive indoloisoquinoline core.
O system in aryloxy substituted isoquinolines would lead to annulation of an indole moiety and construction of the attractive indoloisoquinoline core.
Herein we describe an elegant and effective synthesis of indoxylisoquinolines through Michael addition/aryl-anion migration domino protocol induced by arynes in 1-aryloxy substituted 3,4-dihydroisoquinolines.
Results and discussion
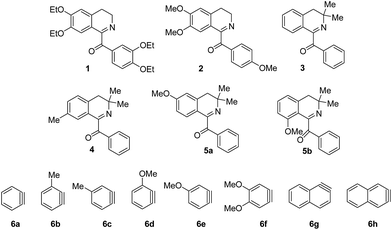
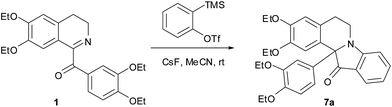
The required for the current study 1-(3,4-diethoxybenzoyl)-6,7-diethoxy-3,4-dihydriosoquinoline 1 was obtained via oxidation of drotaverine by atmospheric oxygen during its isolation from the commercially available hydrochloride; compound 2 was also received by oxidation protocol;12 1-benzoyl-3,3-dimethyl-substituted analogs 3–5 were synthesized by the literature methods13 (Fig. 2). Compounds 5a and 5b were obtained from 3-methoxyphenyl 2-methylpropan-2-ol and represent an inseparable mixture of regioisomers. Arynes 6a–h were studied in present work.Based on the literature data the most used condition for generation of arynes is CsF in acetonitrile at room temperature. We conducted the model experiment with drotaveraldine 1 and benzyne 6a at aforesaid conditions. To our delight the reaction proceeded smoothly to furnish an unusual indoxylisoquinoline 7a in 90% yield (Scheme 1).
In order to minimize the reaction time we tried some other conditions for aryne generation (see ESI for details†), but however the yields were comparable or lower, except benzyne 6h (entry 8), which gave indoxylisoquinoline 7h with good yields only in case of microwave irradiation.
Further studies of scope and limitations of the reaction were done in MeCN in the presence of CsF at room temperature, unless otherwise defined (Table 1).
| Entry | DIQa | Aryne | Product | Yield, % |
|---|---|---|---|---|
| a DIQ-dihydroisoquinoline.b Only one isomer of possible two isomers was isolated.c CsF, THF, 125 °C, μW were used. | ||||
| 1 | 1 | 6a |  |
90 |
| 2 | 1 | 6b |  |
70 |
| 3 | 1 | 6c |  |
75 |
| 4 | 1 | 6d | 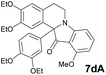 |
67b |
| 5 | 1 | 6e | 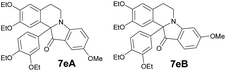 |
80 |
| 6 | 1 | 6f | 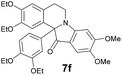 |
85 |
| 7 | 1 | 6g | 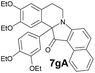 |
87b |
| 8 | 1 | 6h | 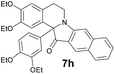 |
67c |
| 9 | 2 | 6a | 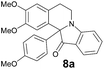 |
73 |
| 10 | 2 | 6f | 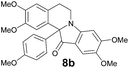 |
77 |
| 11 | 3 | 6a | 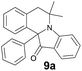 |
93 |
| 12 | 3 | 6c | 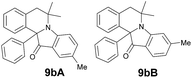 |
91 |
| 13 | 3 | 6f | 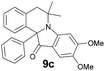 |
94 |
| 14 | 3 | 6g | 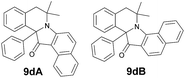 |
72 |
| 15 | 4 | 6a | 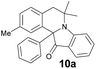 |
69 |
| 16 | 4 | 6f | 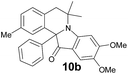 |
87 |
| 17 | 5a | 6a | 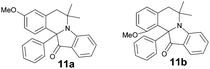 |
a: 57 |
| 5b | b: 21 | |||
The reaction proceeds smoothly and well tolerates the substituents in aryne moiety. In case of compound 3 the target indoxylisoquinolines were formed faster compared to ethoxy- and methoxy substituted analogs and were precipitated from reaction mixtures.
It is noteworthy, that the unsymmetrical arynes 6d and 6g regioselectivity gave only one isomer. The regioselectivity could be explained in terms of the electronic and steric factors that favour the nucleophilic attack on the less hindered position.3j,10h,14
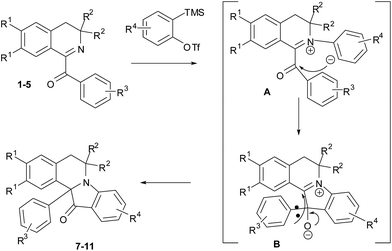
Based on the literature data3f,4g,5f,10a,b,h,j,11 we suppose that the reaction starts with the nucleophilic attack of the isoquinoline nitrogen on generated in situ aryne leading to zwitterion A, the anionic center of which attacks further carbonyl group, giving oxy-anion B. Re-formation of the carbonyl group with subsequent migration of aryl substituent to C-12a-position leads to formation of indoloisoquinolinones 7–11 (Scheme 2).
Migration of aryl anions during rearrangements of oxy-anions has been reported. Benzylic rearrangements of α-diarylketones through the action of bases15 and quasi-Favorskii rearrangements of α-haloarylketones16 are some examples.
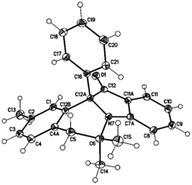
The structure of the product 10a was unambiguously established by X-ray diffraction study and is shown in Fig. 3 along with the atomic numbering schemes.
All synthesized in present work compounds displayed fluorescent properties both in solid state and in solution. Recently the fluorescent properties of indoloisoquinoline system were described.17 In order to investigate the optical properties of obtained compounds the absorbance and emission spectra were recorded. It was found that the solutions of our indoxylisoquinolines are characterized by green-shifted spectra, except compound 7h, that demonstrated more red shift. The quantum yield with the use of p-HOBDI-BF2 (ref. 18) as a standard was calculated (Table 2).
| Compound | Abs | Em | QY |
|---|---|---|---|
| a Peak maximum in nm (extinction coefficient in (mol cm)−1). | |||
| 7a | 399 (4200)a | 474 | 0.24 |
| 7dA | 389 (3600) | 466 | 0.22 |
| 7f | 398 (6300) | 479 | 0.26 |
| 7h | 463 (1700) | 581 | 0.26 |
| 8a | 399 (3900) | 478 | 0.38 |
| 8b | 397 (5500) | 481 | 0.42 |
| 9a | 414 (4100) | 465 | 0.23 |
| 9c | 413 (5500) | 476 | 0.36 |
| 9dA | 423 (4400) | 467 | 0.26 |
| 10a | 413 (3800) | 464 | 0.42 |
| 10b | 413 (4800) | 477 | 0.26 |
| 11a | 413 (3600) | 465 | 0.25 |
Conclusions
In summary, we developed a facile and efficient method for the synthesis of a new class of fluorescent 12a-aryl substituted indoxylisoquinolines through the interaction of arynes with 1-aroyl-3,4-dihydroisoquinolines resulted in annulation of an indolinone moiety that was accompanied by a shift of the aryl substituent from the isoquinoline 1-carbonyl. To the best of our knowledge this is the first example of the aryne-induced aryl anion migration. Further studies on the application of the present methodology along with the investigation of biological properties are in progress.Acknowledgements
This work was supported by RFBR grant No. 14-03-00311. This research was carried out using equipment provided by the IBCH core facility (CKP IBCH).Notes and references
- (a) A. V. Dubrovskiy, N. A. Markina and R. C. Larock, Org. Biomol. Chem., 2013, 11, 191–218 RSC; (b) C. Wu and F. Shi, Asian J. Org. Chem., 2013, 2, 116 CrossRef CAS; (c) A. Bhunia, S. R. Yetra and A. T. Biju, Chem. Soc. Rev., 2012, 41, 3140–3152 RSC; (d) C. M. Gampe and E. M. Carreira, Angew. Chem. Int. Ed., 2012, 51, 3766–3778 (Angew. Chem., 2012, 124, 3829–3842) CrossRef CAS PubMed; (e) H. Pellissier and M. Santelli, Tetrahedron, 2003, 59, 701–730 CrossRef CAS; (f) R. W. Hoffmann, in Dehydrobenzene and Cycloalkynes, Academic, New York, 1967 Search PubMed; (g) H. Hart, in The Chemistry of Triple-bonded Functional Groups Supplement C2, ed. S. Patai, Wiley, New York, 1994, pp. 1017–1134 Search PubMed.
- Y. Himeshima, T. Sonoda and H. Kobayashi, Chem. Lett., 1983, 1211 CrossRef CAS.
- (a) D. C. Rogness and R. C. Larock, J. Org. Chem., 2010, 75, 2289–2295 CrossRef CAS PubMed; (b) K. R. Buszek, N. Brown and D. Luo, Org. Lett., 2009, 11, 201–204 CrossRef CAS PubMed; (c) E. Yoshioka, H. Tamenaga and H. Miyabe, Tetrahedron Lett., 2014, 55, 1402–1405 CrossRef CAS; (d) W.-G. Shou, Y.-Y. Yang and Y.-G. Wang, J. Org. Chem., 2006, 71, 9241–9243 CrossRef CAS PubMed; (e) S. S. Bhojgude, M. Thangaraj, E. Suresh and A. T. Biju, Org. Lett., 2014, 16, 3576–3579 CrossRef CAS PubMed; (f) J.-C. Castillo, J. Quiroga, R. Abonia, J. Rodriguez and Y. Coquerel, Org. Lett., 2015, 17, 3374–3377 CrossRef CAS PubMed; (g) L. Wu, H. Huang, P. Dang, Y. Liang and S. Pi, RSC Adv., 2015, 5, 64354–64357 RSC; (h) S. Su, N. Wang, C. Li, B. Song, X. Jia and J. Li, Asian J. Org. Chem., 2014, 3, 269–272 CrossRef CAS; (i) F. Sha, Y. Tao, C.-Y. Tang, F. Zhang and X.-Y. Wu, J. Org. Chem., 2015, 80, 8122–8133 CrossRef CAS PubMed; (j) Y. Tao, F. Zhang, C.-Y. Tang, X.-Y. Wu and F. Sha, Asian J. Org. Chem., 2014, 3, 1292–1301 CrossRef CAS.
- (a) M. Dai, Z. Wang and S. J. Danishefsky, Tetrahedron Lett., 2008, 49, 6613–6616 CrossRef CAS PubMed; (b) C. Spiteri, P. Sharma, F. Zhang, S. J. F. Macdonald, S. Keeling and J. E. Moses, Chem. Commun., 2010, 46, 1272–1274 RSC; (c) A. V. Dubrovskiy and R. C. Larock, Org. Lett., 2010, 12, 1180–1183 CrossRef CAS PubMed; (d) J. A. Crossley and D. L. Browne, Tetrahedron Lett., 2010, 51, 2271–2273 CrossRef CAS; (e) F. Shi, R. Mancuso and R. C. Larock, Tetrahedron Lett., 2009, 50, 4067–4070 CrossRef CAS PubMed; (f) A. Kivrak and R. C. Larock, J. Org. Chem., 2010, 75, 7381–7387 CrossRef CAS PubMed; (g) C. Spiteri, S. Keeling and J. E. Moses, Org. Lett., 2010, 12, 3368–3371 CrossRef CAS PubMed; (h) B. V. Subba Reddy, K. Praneeth and J. S. Yadav, Carbohydr. Res., 2011, 346, 995–998 CrossRef CAS PubMed.
- (a) See review: P. M. Tadross and B. M. Stoltz, Chem. Rev., 2012, 112, 3550–3577 CrossRef CAS PubMed; (b) X. Huang and T. Zhang, J. Org. Chem., 2010, 75, 506–509 CrossRef CAS PubMed; (c) Ch. D. Gilmore, K. M. Allan and B. M. Stoltz, J. Am. Chem. Soc., 2008, 130, 1558–1559 CrossRef CAS PubMed; (d) D. C. Rogness, N. A. Markina, J. P. Waldo and R. C. Larock, J. Org. Chem., 2012, 77, 2743–2755 CrossRef CAS PubMed; (e) D. Hong, Z. Chen, X. Lin and Y. Wang, Org. Lett., 2010, 12, 4608–4611 CrossRef CAS PubMed; (f) S. D. Vaidya and N. P. Argade, Org. Lett., 2013, 15, 4006–4009 CrossRef CAS PubMed.
- (a) T. Kametani and K. J. Ogasawara, J. Chem. Soc., 1967, 2208 CAS; (b) K. Orito, R. Harada, S. Uchiito and M. Tokuda, Org. Lett., 2000, 2, 1799–1801 CrossRef CAS PubMed; (c) A. N. C. Lötter, R. Pathak, T. S. Sello, M. A. Fernandes, W. A. L. van Otterlo and C. B. de Koning, Tetrahedron, 2007, 63, 2263–2274 CrossRef.
- (a) R. Ambros, S. von Angerer and W. Wiegrebe, Arch. Pharm., 1988, 321, 743–747 CrossRef CAS; (b) R. Ambros, M. R. Schneider and S. Von Angerer, J. Med. Chem., 1990, 33, 153–160 CrossRef CAS PubMed; (c) T. Polossek, R. Ambros, S. von Angerer, G. Brandl, A. Mannschreck and E. von Angerer, J. Med. Chem., 1992, 35, 3537–3547 CrossRef CAS PubMed; (d) M. Goldbrunner, G. Loidl, T. Polossek, A. Mannschreck and E. von Angerer, J. Med. Chem., 1997, 40, 3524–3533 CrossRef CAS PubMed; (e) G. A. Kraus, V. Gupta, M. Kohutb and N. Singh, Bioorg. Med. Chem. Lett., 2009, 19, 5539–5542 CrossRef CAS PubMed.
- (a) E. Valencia, A. J. Freyer and M. Shamma, Tetrahedron Lett., 1984, 25, 599–602 CrossRef CAS; (b) A. Moreau, A. Couture, E. Deniau, P. Grandclaudon and S. Lebrun, Tetrahedron, 2004, 60, 6169–6176 CrossRef CAS; (c) A. Moreau, A. Couture, E. Deniau and P. Grandclaudon, Eur. J. Org. Chem., 2005, 3437–3442 CrossRef CAS; (d) J. Selvakumara and Ch. R. Ramanathan, Org. Biomol. Chem., 2011, 9, 7643–7646 RSC.
- (a) M. Winn and H. E. Zaugg, J. Org. Chem., 1968, 33, 3779–3783 CrossRef CAS; (b) G. J. Hitchings, M. D. Thomas and J. M. Vernon, J. Chem. Soc., Perkin Trans. 1, 1992, 895–898 RSC; (c) J.-R. Huang, L. Qin, Y.-Q. Zhu, Q. Song and L. Dong, Chem. Commun., 2015, 51, 2844–2847 RSC.
- (a) A. Bhunia, T. Roy, P. Pachfule, P. R. Rajamohanan and A. T. Biju, Angew. Chem. Int. Ed., 2013, 52, 10040–10043 (Angew. Chem., 2013, 125, 10224–10227) CrossRef CAS PubMed; (b) A. Bhunia, D. Porwal, R. G. Gonnade and A. T. Biju, Org. Lett., 2013, 15, 4620–4623 CrossRef CAS PubMed; (c) M. Jeganmohan and C.-H. Cheng, Chem. Commun., 2006, 2454–2456 RSC; (d) C. E. Hendrick and Q. Wang, J. Org. Chem., 2015, 80, 1059–1069 CrossRef CAS PubMed; (e) J. Wallbaum, P. G. Jones and D. B. Werz, J. Org. Chem., 2015, 80, 3730–3734 CrossRef CAS PubMed; (f) J. Shi, D. Qiu, J. Wang, H. Xu and Y. Li, J. Am. Chem. Soc., 2015, 137, 5670–5673 CrossRef CAS PubMed; (g) Z. Liu, F. Shi, P. D. G. Martinez, C. Raminelli and R. C. Larock, J. Org. Chem., 2008, 73, 219–226 CrossRef CAS PubMed; (h) S. D. Vaidya and N. P. Argade, Org. Lett., 2013, 15, 4006–4009 CrossRef CAS PubMed; (i) A. Bunescu, C. Piemontesi, Q. Wang and J. Zhu, Chem. Commun., 2013, 49, 10284–10286 RSC; (j) D. K. Rayabarapu, K. K. Majumdar, T. Sambaiah and C.-H. Chen, J. Org. Chem., 2001, 66, 3646–3649 CrossRef CAS PubMed; (k) F. Nawaz, K. Mohanan, L. Charles, M. Rajzmann, D. Bonne, O. Chuzel, J. Rodriguez and Y. Coquerel, Chem.–Eur. J., 2013, 19, 17578–17583 CrossRef CAS PubMed.
- (a) D. Giallombardo, A. C. Nevin, W. Lewis, C. C. Nawrat, R. R. A. Kitson and C. J. Moodya, Tetrahedron, 2014, 70, 1283–1288 CrossRef CAS; (b) J. Zhao and R. C. Larock, Org. Lett., 2005, 7, 4273–4275 CrossRef CAS PubMed; (c) J. Zhao and R. C. Larock, J. Org. Chem., 2007, 72, 583–588 CrossRef CAS PubMed; (d) A. Bunescu, Q. Wang and J. Zhu, Org. Lett., 2014, 16, 1756–1759 CrossRef CAS PubMed; (e) D. C. Rogness and R. C. Larock, J. Org. Chem., 2011, 76, 4980–4986 CrossRef CAS PubMed; (f) F. Shi, R. Mancuso and R. C. Larock, Tetrahedron Lett., 2009, 50, 4067–4070 CrossRef CAS PubMed; (g) C.-Y. Tang, G. Wang, X.-Y. Yang, X.-Y. Wu and F. Sha, Tetrahedron Lett., 2014, 55, 6447–6450 CrossRef CAS; (h) M. Jeganmohan, S. Bhuvaneswari and C.-H. Cheng, Chem.–Asian J., 2010, 5, 153–159 CrossRef CAS PubMed.
- R.-Y. Kuo, F.-R. Chang, C.-C. Wu, R. Patnam, W.-Y. Wang, Y.-C. Du and Y.-C. Wu, Bioorg. Med. Chem. Lett., 2003, 13, 2789–2793 CrossRef CAS PubMed.
- A. G. Mikhailovskii, Chem. Heterocycl. Compd., 2000, 36, 223 CrossRef CAS.
- Z. Liu, F. Shi, P. D. G. Martinez, C. Raminelli and R. C. Larock, J. Org. Chem., 2008, 73, 219–226 CrossRef CAS PubMed.
- (a) E. Q. Selman, Chem. Soc. Rev., 1960, 14, 221–235 RSC; (b) B. Fohlishen, A. Radl, R. Schwetzler-Raschke and S. Henkel, Eur. J. Org. Chem., 2001, 4357–4365 Search PubMed.
- (a) M. Harmata, G. Bohner, L. Kurti and C. L. Barnes, Tetrahedron Lett., 2002, 43, 2347–2349 CrossRef CAS; (b) S. S. Bordwell, J. Am. Chem. Soc., 1969, 91, 2087–2093 CrossRef.
- H. Sun, C. Wang, Y.-F. Yang, P. Chen, Y.-D. Wu, X. Zhang and Y. Huang, J. Org. Chem., 2014, 79, 11863–11872 CrossRef CAS PubMed.
- M. S. Baranov, K. A. Lukyanov, A. O. Borissova, J. Shamir, D. Kosenkov, L. V. Slipchenko, L. M. Tolbert, I. V. Yampolsky and K. M. Solntsev, J. Am. Chem. Soc., 2012, 134, 6025–6032 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, copies of 1H and 13C spectra. CCDC 1043893. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra25323c |
| This journal is © The Royal Society of Chemistry 2016 |