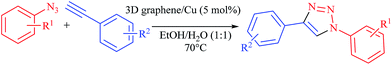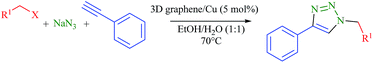Copper nanoparticle decorated three dimensional graphene with high catalytic activity for Huisgen 1,3-dipolar cycloaddition†
Minoo Dabiri*,
Melika Kasmaei,
Parinaz Salari and
Siyavash Kazemi Movahed
Faculty of Chemistry, Shahid Beheshti University, Tehran 1983969411, Islamic Republic of Iran. E-mail: m-dabiri@sbu.ac.ir
First published on 10th June 2016
Abstract
A copper nanoparticle decorated three-dimensional graphene nanocomposite was prepared at room temperature by reduction of copper sulfate using L-ascorbic acid as the reducing agent. The synthesized Cu NPs-3D G nanocatalyst exhibited excellent catalytic activity in the synthesis of 1,2,3-triazoles.
Graphene is a two-dimensional sheet of sp2 bonded carbon atoms, which can be viewed as an extra-large polycyclic aromatic molecule. It has been used as the support for metal and metal oxides mainly because of its large surface area, superior electrical and thermal conductivity, low price, high chemical inertness, easy modification and robust interactions with metal clusters.1 Recently, three-dimensional (3D) graphene, a framework of connecting graphene sheets, has attracted great interest in energy storage/conversion, as an absorbent of contaminants, in catalysis, in composites, and in chemical sensors due to its ultrahigh surface-to-volume ratio, high porosity, strong mechanical strength, excellent electrical conductivity and fast mass and electron transport kinetics.2
1,2,3-Triazoles have received considerable interest because of their useful applications as agrochemicals, corrosion inhibitors, dyes, optical brighteners as well as biologically active agents.3 The well-established approach utilized thus far for the synthesis of the 1,2,3-triazole ring system relies on the thermal 1,3-dipolar Huisgen cycloaddition between alkynes and azides. However, this non-catalyzed process exhibits several disadvantages, including: (i) the requirement for high-temperature conditions which may cause the decomposition of labile products, (ii) low yields, (iii) poor regioselectivity which leads to a mixture of 1,4- and 1,5-disubstituted triazoles.4 Thus to overcome these drawbacks, an improved procedure involving Cu(I)-catalyzed azide–alkyne 1,3-dipolar cycloaddition has been reported,5 which affords high regioselectivity at room temperature reaction. Because of their thermodynamic instability and initiation of the undesired alkyne–alkyne coupling, the direct use of Cu(I) salt is restricted while, in situ reduction of Cu(II) to Cu(0), copper(II)/copper(0) comproportionation avoids such side reactions.5,6
Based on this introduction and also as part of our ongoing studies on the synthesis of graphene-based nanocomposite catalysts,7 herein, we report a novel protocol for the Huisgen 1,3-dipolar cycloaddition by free-standing 3D graphene/Cu nanocomposite which is prepared by reduction of copper nitrate using L-ascorbic acid as reducing agent onto the 3D graphene framework. Additionally, the effects of solvent polarity, and temperature on the yield, and recycling potential of the catalyst all have been assessed.
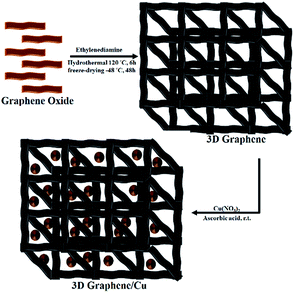
The first, graphene oxide (GO) was prepared from natural graphite by modified Hummers method.8 The dispersed GO was mixed uniformly with ethylenediamine (EDA). The resulting stable suspension was transferred into a Teflon-lined autoclave and heated for 6 h at 120 °C for the synthesis of 3D graphene hydrogel.9 After freeze-drying, the 3D graphene aerogel was produced. Then, decoration of Cu NPs was performed on 3D graphene by the reduction of copper nitrate using L-ascorbic acid as the reducing agent (Scheme 1).
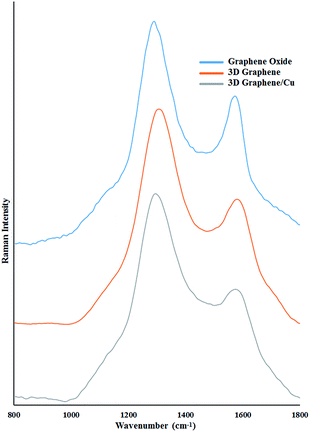
The 3D graphene/Cu nanocomposite was characterized by Raman, XRD, SEM and TEM measurements. Raman spectroscopy is a very useful tool for investigating the electronic and phonon structure of graphene-based materials.10 The Raman spectra of the prepared 3D graphene and the 3D graphene/Cu nanocomposite are shown in Fig. 1. As can be seen in Fig. 1, after hydrothermal synthesis of the GO with the EDA, the ID/IG was 1.71 for the resulting 3D graphene, while the ID/IG of the GO was 1.50. The higher value of ID/IG observed in 3D graphene clearly demonstrates the heteroatomic doping of nitrogen into the graphene frame and the enhanced degree of disorder.11 The ratio ID/IG increases to 1.85 for the 3D graphene/Cu. Such an enhancement has also been observed for metal nanoparticle composites of graphene, indicating a probable chemical interaction or bond between the metal nanoparticles and graphene.12
X-ray diffraction (XRD) patterns of graphene oxide, 3D graphene and 3D graphene/Cu nanocomposite are displayed in Fig. 2. It can be seen that the GO shows a strong diffraction peak centered at 2θ = 10.9° corresponding to the (001) lattice plane with interlayer spacing of 0.80 nm resulting from the insertion of hydroxyl and epoxy groups between the graphite sheets as a result of the oxidation process of graphite.13 However, the peak at 2θ = 10.9° entirely collapses after hydrothermal reaction, and a broad diffraction peak around 2θ = 19–30° of the graphite plane (002) is observed for the synthesized 3D graphene sample, indicating that the framework of the reduced sample is composed of few-layer stacked graphene sheets.14 Besides, the interlayer distance is reduced from 0.80 nm of the GO to 0.35 nm of the 3D graphene, which certifies the recovery of the π-conjugated system after the reduction of the GO.15 In the XRD pattern of the Cu@Cu2O NPs–RGO the peaks at 2θ = 43.1°, 50.2°, 73.8° can be assigned to the (111), (200), (220) lattice planes of Cu(0) that are consistent with the presence of face-centred cubic copper (JCPDS No. 4-0836) and also three diffraction peaks 2θ = 36.2°, 42.0° and 61.2° can be assigned to the (111), (200) and (220) lattice planes of Cu2O (PDF No. 01-078-2076). From these results, it is inferred that the copper nanoparticles is oxidized. Additionally, the peaks at 2θ = 44.1 and 64.4° correspond to stainless steel sample holder of powder diffractometer.
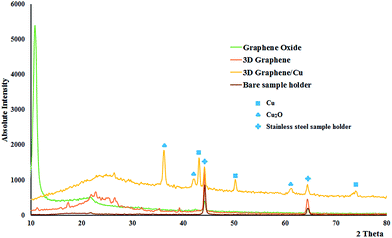 | ||
| Fig. 2 XRD patterns of graphene oxide, 3D graphene, 3D graphene/Cu nanocomposite and bare sample holder. | ||
As shown in ESI, Fig. S3†, the Brunauer–Emmett–Teller (BET) specific surface area is 17.68 m2 g−1. This value is smaller than that of 3D graphene (68.59 m2 g−1), indicating the presence of Cu nanoparticles decreases the surface area of the product.16 The pore volume determined by the Barrett–Joyner–Halenda (BJH) method is 0.03 cm3 g−1 for the product. This value is smaller than that of the 3D graphene (0.12 cm3 g−1).
The XPS spectrum of Cu 2p core level region for 3D graphene/Cu nanocomposite displays main peaks at 932.97 eV and 953.78 eV which are attributed to the binding energy of Cu 2p3/2 and Cu 2p1/2, respectively (Fig. S4†). The broad Cu 2p3/2 peak has been deconvoluted into two peaks which are marked as peaks at 932.94 eV and 935.20 eV, and assigned to Cu2O/Cu (Cu1+/Cu0) and CuO/Cu(OH)2 (Cu2+), respectively. Actually, it is difficult to differentiate Cu2O (Cu1+) and Cu0 by the XPS feature of Cu 2p3/2 as the binding energies of Cu and Cu2O are very close and different by only 0.1–0.2 eV. One can distinguish them from the position of their LMM auger transition in the XPS spectra which are 568 eV and 570 eV for Cu and Cu2O, respectively.17 There is a peak at about 570.17 eV in the XPS spectra of the 3D graphene/Cu nanocomposite. According to above mentioned fact, one can conclude that the peak at 932.94 eV is related to Cu2O. Also, a shake-up satellite at 944.20 eV was observed in the XPS spectrum. This peak is typically associated with copper in the bivalent oxidation state, which is assigned to Cu2+ in CuO (or probably Cu(OH)2 species). From these results it is inferred that the surface of copper nanoparticles is oxidized.
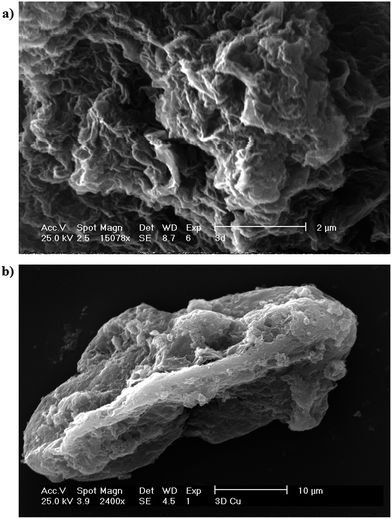
The 3D morphology of as-prepared 3D graphene is confirmed by scanning electron microscopy (SEM) characterization (Fig. 3a). The 3D graphene/Cu exhibits a foam-like structure with interconnected pores.
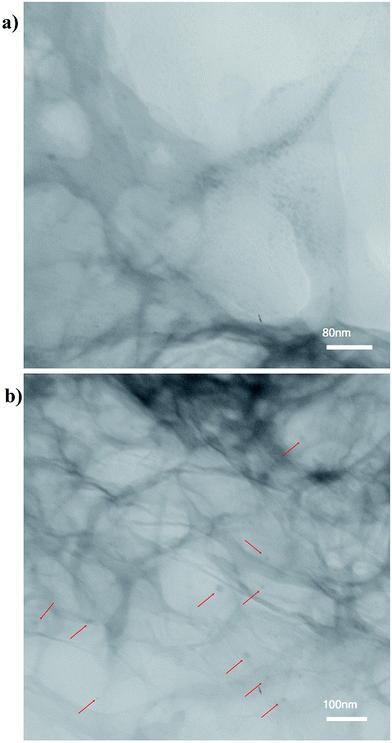
Transmission electron microscopy (TEM) observation reveals that the surfaces of 3D graphene are covered with distributed Cu NPs with an average size of 5–15 nm (Fig. 4a and b).
After careful investigation of the prepared 3D graphene/Cu nanocomposite, it was tested for catalyzing 1,3-dipolar Huisgen cycloaddition between terminal alkynes and aryl azides.
To optimize the reaction conditions, ethynylbenzene, 1-azido-4-nitrobenzene were tested as model substrates in the presence of various solvents (Table 1). The results indicate that both reaction temperature and solvent significantly influence the product yield. It was also notable that, when this reaction was carried out with 3D graphene, cycloaddition products were isolated without regioselectivity (1,4 and 1,5 regioisomers) (Scheme 2).
| Entry | Solvent | Temp. (°C) | Yieldb (%) |
|---|---|---|---|
| a Ethynylbenzene (1 mmol), 1-azido-4-nitrobenzene (1 mmol), solvent (3 mL), 1 h, 3D graphene/Cu nanocomposite (5 mol%).b Isolated yield. | |||
| 1 | Ethanol | 70 | 89 |
| 2 | Water | 70 | 82 |
| 3 | Ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1 water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
70 | 99 |
| 4 | Methanol | Reflux | 90 |
| 6 | CH2Cl2 | Reflux | 76 |
| 7 | CH3CN | Reflux | 83 |
| 8 | Ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1 water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
60 | 82 |
| 9 | Ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1 water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 99 |
Using the optimized reaction conditions, we attempted to expand the scope of terminal alkynes and aryl azides in water at 70 °C, using 3D graphene/Cu nanocomposite (5 mol% of Cu) (Table 2). Electron donating substituents like methyl (Entry 2, Table 2), and electron withdrawing substituents such as trifluoromethyl at para position of ethynylbenzene (Entry 3, Table 2) reacted well and gave good yields.
Inspired by the high activity and stability of the 3D graphene/Cu nanocomposite, the multicomponent 1,3-dipolar cycloaddition of terminal alkynes and organic azides (generated in situ from sodium azide and different organic halides) were further employed as another model reaction to test the high performance of the 3D graphene/Cu nanocatalyst. Using the optimized reaction conditions, we attempted to expand the scope of organic halides and terminal alkynes in this reaction (Table 3). Different substituents like bromide and nitro at para position of benzyl bromide (Entries 2 and 3, Table 3) were equally effective toward the nucleophilic substitution of azide, followed by 1,3-dipolar cycloaddition. However, in the case of non-activated alkyl halide like n-decyl bromide, the reaction required longer reaction time and furnished corresponding triazole in lower yield (Entry 4, Table 3). Unfortunately, the catalytic system was less effective for the reaction of benzyl chloride (Entry 5, Table 3).
One of the main criteria for using heterogeneous catalysts from an industrial perspective is the recovery and reusability of the catalyst. To check the reusability of the catalyst, we performed the 1,3-dipolar Huisgen cycloaddition under optimized conditions on a 4 mmol scale of ethynylbenzene. After 1 h, the catalyst was centrifuged and washed with excess amount of ethyl acetate and water. Finally, the recovered catalyst was dried at room temperature. The catalyst was then reused in another ten catalytic cycles under identical reaction conditions, and the results showed good conversion with a slight decrease in the selectivities for the cycloaddition products (Table 4); the Cu content of the reused catalyst (after the tenth run) was 2.58%, as obtained by ICP-OES. This showed that leaching of Cu was very low, thus, the catalyst could be reused without any significant changes in the products yield.
Moreover, no appreciable changes in textural properties after ten reactions cycles were detected, as is clearly evidenced from the TEM analysis of the recycled catalyst (Fig. 5). The TEM image also indicates a few aggregation of Cu NPs in the recovered 3D graphene/Cu nanocomposite after 10th reaction run.
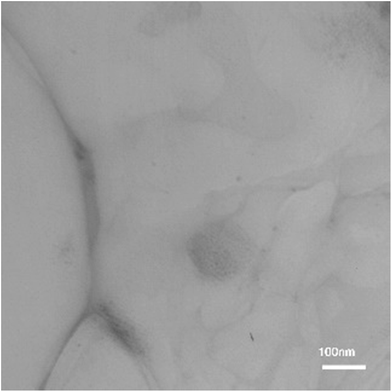 | ||
| Fig. 5 TEM image of 3D graphene/Cu nanocomposite reused after ten cycles in 1,3-dipolar Huisgen cycloaddition between of ethynylbenzene with 1-azido-4-nitrobenzene. | ||
Conclusions
A 3-D hybrid material has been developed by the Cu NP immobilization on 3D graphene framework. The prepared 3D graphene/Cu nanocomposite exhibits a high catalytic activity for the 1,3-dipolar cycloaddition of aryl azides and terminal alkynes and the 1,3-dipolar cycloaddition of alkyl/benzyl halides, sodium azide and terminal alkynes in the aqueous medium. Moreover, the catalyst is chemically stable and can be recycled for at least ten times in the corresponding reaction without reduction in the catalytic activity.Acknowledgements
We gratefully acknowledge financial support from the Research Council of Shahid Beheshti University and the Iranian National Science Foundation (Proposal No. 94809515).References
- B. F. Machado and P. Serp, Catal. Sci. Technol., 2012, 2, 54 Search PubMed; S. Bai and X. Shen, RSC Adv., 2012, 2, 64 RSC; S. K. Movahed, M. Fakharian, M. Dabiri and A. Bazgir, RSC Adv., 2014, 4, 5243 RSC.
- C. Hu, Z. Mou, G. Lu, N. Chen, Z. Dong, M. Hu and L. Qu, Phys. Chem. Chem. Phys., 2013, 15, 13038 RSC; X. Zhu, P. Zhang, S. Xu, X. Yan and Q. Xue, ACS Appl. Mater. Interfaces, 2014, 6, 11665 Search PubMed.
- G. C. Tron, T. Pirali, R. A. Billington, P. L. Canonico, G. Sorba and A. A. Genazzani, Med. Res. Rev., 2008, 28, 278 CrossRef CAS PubMed; C. D. Hein, X.-M. Liu and D. Wang, Pharm. Res., 2008, 25, 2216 CrossRef PubMed.
- H. A. Orgueira, D. Fokas, Y. Isome, P. C.-M. Chan and C. M. Baldino, Tetrahedron Lett., 2005, 46, 2911 CrossRef CAS.
- N. Mukherjee, S. Ahammed, S. Bhadra and B. C. Ranu, Green Chem., 2013, 15, 389 RSC; K. Jayaramulu, V. M. Suresh and T. K. Maji, Dalton Trans., 2015, 44, 83 RSC; R. B. N. Baig and R. S. Varma, Green Chem., 2012, 14, 625 RSC; F. Alonso, Y. Moglie, G. Radivoy and M. Yus, Adv. Synth. Catal., 2010, 352, 3208 CrossRef CAS; I. S. Park, M. S. Kwon, Y. Kim, J. S. Lee and J. Park, Org. Lett., 2008, 10, 497 CrossRef PubMed; F. Nador, M. A. Volpe, F. Alonso, A. Feldhoff, A. Kirschning and G. Radivoy, Appl. Catal., A, 2013, 455, 39 CrossRef.
- M. Meldal and C. W. Tornøe, Chem. Rev., 2008, 108, 2952 CrossRef CAS PubMed.
- S. K. Movahed, N. F. Lehi and M. Dabiri, RSC Adv., 2014, 4, 42155 RSC; S. K. Movahed, R. Esmatpoursalmani and A. Bazgir, RSC Adv., 2014, 4, 14586 RSC; S. K. Movahed, M. Shariatipour and M. Dabiri, RSC Adv., 2015, 5, 33423 RSC; M. Dabiri, M. Shariatipour, S. K. Movahed and S. Bashiribod, RSC Adv., 2014, 4, 39428 RSC.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS; N. I. Kovtyukhova, P. J. Ollivier, B. R. Martin, T. E. Mallouk, S. A. Chizhik, E. V. Buzaneva and A. D. Gorchinskiy, Chem. Mater., 1999, 11, 771 CrossRef.
- H. Hu, Z. B. Zhao, W. B. Wan, Y. Gogotsi and J. S. Qiu, Adv. Mater., 2013, 25, 2219 CrossRef CAS PubMed.
- A. Jorio, M. S. Dresselhaus, R. Saito and G. F. Dresselhaus, in Raman Spectroscopy in Graphene Related Systems, Wiley-VCH, Berlin, 2011 Search PubMed.
- Z.-Y. Yang, Y.-X. Zhang, L. Jing, Y.-F. Zhao, Y.-M. Yan and K.-N. Sun, J. Mater. Chem. A, 2014, 2, 2623 CAS.
- K. Jasuja and V. Berry, ACS Nano, 2009, 3, 2358 CrossRef CAS PubMed; K. Jasuja, J. Linn, S. Melton and V. Berry, J. Phys. Chem. Lett., 2010, 1, 1853 CrossRef; Y. Li, X. Fan, J. Qi, J. Ji, S. Wang, G. Zhang and F. Zhang, Nano Res., 2010, 3, 429 CrossRef.
- C.-W. Tsai, M.-H. Tu, C.-J. Chen, T.-F. Hung, R.-S. Liu, W.-R. Liu, M.-Y. Lo, Y.-M. Peng, L. Zhang, J. Zhang, D.-S. Shyd and X.-K. Xing, RSC Adv., 2011, 1, 1349 RSC.
- D. H. Long, W. Li, L. C. Ling, J. Miyawaki, I. Mochida and S. H. Yoon, Langmuir, 2010, 26, 16096 CrossRef CAS PubMed.
- L. Sun, L. Wang, C. Tian, T. Tan, Y. Xie, K. Shi, M. Li and H. Fu, RSC Adv., 2012, 2, 4498 RSC.
- W. Chen, S. Li, C. Chen and L. Yan, Adv. Mater., 2011, 23, 5679 CrossRef CAS PubMed.
- T. Ghodselahi, M. A. Vesaghi, A. Shafiekhani, A. Baghizadeh and M. Lameii, Appl. Surf. Sci., 2008, 255, 2730 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra25317a |
| This journal is © The Royal Society of Chemistry 2016 |