Quantum dot cluster (QDC)-loaded phospholipid micelles as a FRET probe for phospholipase A2 detection†
Abstract
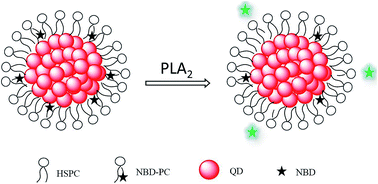
A simple assay for phospholipase A2 (PLA2) enzyme was developed based on a fluorescence resonance energy transfer (FRET) probe using the quantum dot cluster (QDC)-loaded phospholipid micelles. The probe was prepared by encapsulating many small hydrophobic quantum dots (QDs) within the hydrophobic core of micelles that were formed from the coassembly of hydrogenated soy phosphatidylcholine phospholipids (HSPC) and fluorescent lipids (NBD-PC). QDCs formed within the micelle core served as the substrate for NBD fluorescence quenching through FRET. The QDC-loaded micelles showed very low background fluorescence. As the PLA2 enzyme selectively digested lipids, the NBD fluorescence was recovered from its quenched state, leading to the sensitive detection of PLA2. This assay provided a limit of detection (at a signal-to-noise ratio of 3) of 3 U L−1 for PLA2. In the presence of a PLA2 inhibitor, the fluorescent response of the sensor for PLA2 decreased, indicating that the assay could also be used for screening the PLA2 inhibitors.

 Please wait while we load your content...
Please wait while we load your content...