Synthesis and characterization of a Cu(II) Schiff base complex immobilized on graphene oxide and its catalytic application in the green synthesis of propargylamines†
Shweta Kumaria,
Amiya Shekharb and
Devendra D. Pathak*a
aDepartment of Applied Chemistry, Indian School of Mines, Dhanbad – 826004, India. E-mail: Shweta@ac.ism.ac.in; amiyashekhar@gmail.com; ddpathak61@gmail.com; Tel: +91 9431126250
bDepartment of Chemistry, Vidya Vihar Institute of Technology, Purnea – 854303, India
First published on 25th January 2016
Abstract
A Cu(II) Schiff base complex immobilized on graphene oxide has been synthesized and characterized by FTIR, FT-RAMAN, XRD, UV, TEM, FE-SEM, EDAX, TGA, N2 adsorption–desorption and AAS analysis. The immobilized complex has been found to be a highly efficient and recyclable heterogeneous catalyst for the synthesis of propargylamines in aqueous medium.
The development of synthetic strategies for C–H bond activation by transition metal catalysts is one of the most important areas in organic chemistry.1 The synthesis of propargylamines by the three-component one-pot coupling reactions (A3-coupling reactions) of aldehydes, alkynes, and secondary amines is one of the best examples of such a process.2 Propargylamines moieties are versatile synthons in the preparation of nitrogen-containing molecules, such as aminoindolizines, β-lactams, oxotremorine analogs, pyrroles, pyrrolidines, isosteres, allylamines, and as key intermediates for natural product synthesis.3,4 The A3-coupling reactions have been reported to be catalysed, by either salts or complexes of transition metals such as gold, silver, ruthenium, copper, iridium, iron, indium, zinc, etc.5,6 However, most of them have certain drawbacks, such as long reaction time, moderate yields, formation of by-products, involvement of expensive reagents and solvents, and use of the microwave radiations.7 The large scale synthesis of propargylamines by the above protocols may entail some environmental & economic concerns. Therefore, the development of novel green heterogeneous catalysts is one of the frontier areas of current research. Herein, our interest lies in exploring new routes for the synthesis of propargylamines using a cost-effective, green and reusable catalyst under mild reaction conditions.
In recent years, graphene oxide (GO) has been demonstrated as a promising, ideal support for number of metals, metal oxides, inorganic complexes, nanoparticles and organic compounds due to its unique nanostructure, high chemical stability and surface area, good accessibility, and functional groups on its basal planes.8 The edges of GO also provide additional advantages.9 Mungse et al. reported the first Schiff base complex of vanadium immobilized on graphene oxide anchored.10 Su et al. prepared Schiff base complexes of transition metals onto graphene oxide for epoxidation of styrene.11 Verma et al. reported oxo-vanadium Schiff base immobilized on graphene oxide as catalyst for the epoxidation of fatty acids and esters.12 Recently, Xiong et al. have reported CuCl2 immobilized on graphene oxide as a catalyst for the synthesis of propargylamines under microwave radiation.7a In continuation of our work on the development of green synthesis methodologies for various organic transformations,13 we herein report the immobilization of a new Cu(II) Schiff base complex onto graphene oxide sheets and its use for the green synthesis of propargylamines. The water is a universal solvent and recently organic reactions in a aqueous medium has attracted a lot of attention.14
The schematic presentation of the synthesis of heterogeneous Cu(II) Schiff base complex immobilized on graphene oxide sheets (GO–Cu) is shown in Scheme 1 (more detail of GO–Cu preparation is given in the ESI†).
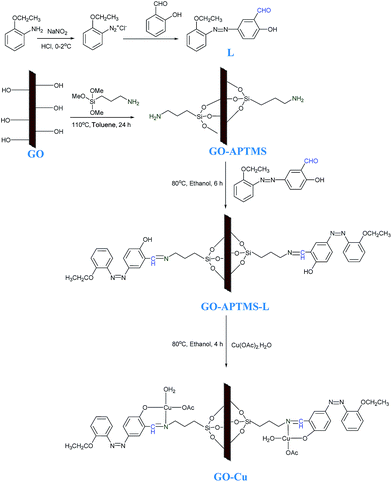 | ||
| Scheme 1 Synthesis of ligand (L) and schematic presentation of the synthesis of Cu(II) Schiff base complex immobilized on graphene oxide sheets. | ||
The chemical changes that occurred during the immobilization of Cu(II) Schiff base complex onto GO were followed by FTIR. The FTIR spectra of GO, GO–APTMS, GO–APTMS-L and GO–Cu are shown in Fig. 1a. The FTIR spectrum of GO exhibited strong characteristics bands at 3419, 1731, 1623, 1223, and 1035.5 cm−1, due to the presence of hydroxyl, phenolic, carbonyl, carboxylic, epoxy, etc. functional groups in the scaffold.15 The FTIR spectrum of GO–APTMS, exhibited a doublet at 2850 and 2917 cm−1, corresponding to the ν symmetric and asymmetric CH2 of the alkyl chains.16a Moreover, the bands observed at 1121.5 and 1034.6 cm−1 were attributed to Si–O–Si and Si–O–C linkages, respectively.16b The bands at 3423.7 and 1573.2 cm−1, due to ν N–H, confirms the successful chemical functionalization of GO sheets.16 The FTIR spectrum of GO–APTMS-L depicted a new peak at 1632 cm−1 due to ν C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond. The formation of C
N bond. The formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N clearly indicated that the condensation reaction between the carbonyl groups of aldehyde of ligand (L) and the –NH2 groups of GO–APTMS had taken place.17 In the FTIR spectrum of GO–Cu, ν C
N clearly indicated that the condensation reaction between the carbonyl groups of aldehyde of ligand (L) and the –NH2 groups of GO–APTMS had taken place.17 In the FTIR spectrum of GO–Cu, ν C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N frequencies were slightly shifted to lower frequencies (Δν = 13 cm−1) at 1619 cm−1, compared with (GO–APTMS-L). The shifting of these absorption bands to lower wave numbers confirmed the coordination of Cu(II) metal ion with azomethine nitrogen atom of the Schiff base.18 However, no change in the ν N
N frequencies were slightly shifted to lower frequencies (Δν = 13 cm−1) at 1619 cm−1, compared with (GO–APTMS-L). The shifting of these absorption bands to lower wave numbers confirmed the coordination of Cu(II) metal ion with azomethine nitrogen atom of the Schiff base.18 However, no change in the ν N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N frequency was observed which rules out the interaction of the azo group to the metal. The new sharp band at 1438 cm−1 was observed, due to the ν CO frequency which, indicated that Cu bonded with –OAc.19a The new bands at 674, 462 cm−1 were attributed to the stretching modes Cu–N and Cu–O bonds, respectively and confirmed the coordination of copper with N, O sites of GO–APTMS-L.19
N frequency was observed which rules out the interaction of the azo group to the metal. The new sharp band at 1438 cm−1 was observed, due to the ν CO frequency which, indicated that Cu bonded with –OAc.19a The new bands at 674, 462 cm−1 were attributed to the stretching modes Cu–N and Cu–O bonds, respectively and confirmed the coordination of copper with N, O sites of GO–APTMS-L.19
 | ||
| Fig. 1 FTIR spectra of (a) GO, GO–APTMS, GO–APTMS-L, GO–Cu and FT-RAMAN spectra of (b) GO, GO–APTMS, GO–APTMS-L, GO–Cu. | ||
The FT-RAMAN spectra of GO, GO–APTMS, GO–APTMS-L, and GO–Cu are shown in Fig. 1b. The FT-RAMAN spectra of GO–Cu complex and other intermediates show two remarkable peaks at around 1351, and 1596 cm−1, which were associated with the well-defined D band and G bands, respectively.8a The values of ID/IG were found to be 0.82, 0.84, 0.87 and 0.89 for GO, GO–APTMS, GO–APTMS-L, and GO–Cu respectively. The gradual increase in the ID/IG ratio is indicative of increasing disorder in the graphene oxide lattice after functionalization.20 The PXRD of GO and GO–Cu are given in Fig. 2. The exfoliated GO exhibited a broad diffraction peak at 2θ = 11.62° with a corresponding d spacing of 0.82 nm, which may due to presence of functionalities in the basal plane of GO along with absorbed water molecules causing increase in the interlayer distance.21a The PXRD spectrum of GO-Cu exhibited a new peak with a very broad pattern around 2θ = 22.24°, closer to reduced graphene (2θ = 24°). The broad diffraction peak of PXRD of GO–Cu indicates that the synthesized composite is almost amorphous in nature.21 The UV-visible spectra of GO (Fig. S1†) and GO–APTMS, ligand, GO–APTMS-L, GO–Cu are shown in Fig. S2† respectively. The UV-vis spectrum of GO depicted a strong absorption peak at 230 nm and a shoulder at about 300 nm, which were attributed to the π/π* transitions of the graphitic C–C bonds and to the n/π* transitions of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively (Fig. S1†).22a However, the UV-vis spectrum of the GO–Cu showed one broad band at 535 nm, due to d–d transitions of the Cu ions (Fig. S2†).22 Micro-structural features and morphology of GO and GO–Cu were analyzed by FESEM (Fig. S3†) and TEM (Fig. 3), FESEM image of GO–APTMS, GO–APTMS-L materials are also shown in the ESI (Fig. S3†).
O bonds, respectively (Fig. S1†).22a However, the UV-vis spectrum of the GO–Cu showed one broad band at 535 nm, due to d–d transitions of the Cu ions (Fig. S2†).22 Micro-structural features and morphology of GO and GO–Cu were analyzed by FESEM (Fig. S3†) and TEM (Fig. 3), FESEM image of GO–APTMS, GO–APTMS-L materials are also shown in the ESI (Fig. S3†).
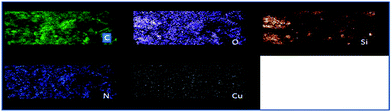
The FESEM images of GO and GO–Cu exhibited twisted nanosheets in disordered phase with a lot of wrinkles and crumpling features (Fig. S3†).23 These folded edges and protrusions, owing to sp3-carbon in GO, bear the Cu immobilised Schiff base complex on both sides of the GO nanosheets (Fig. S3d†). These sites may serve as ideal templates for reactant molecules to accomplish the desired transformation.16 The TEM images of GO (Fig. 3a) revealed the nanoscopic features with few numbers of layers. The Fig. 3b also depicted fine nanostructures of the GO–Cu. The EDAX analysis provides detailed chemical analysis on the GO, GO–APTMS, GO–APTMS-L and GO–Cu (Fig. S4†). The GO was found to be mainly composed of carbon and oxygen. The loading of Cu(II) Schiff base complex on GO, changed the elemental composition with the introduction of copper, nitrogen and silicon, which are core elements of the complex apart from carbon and oxygen. Further, EDAX elemental mapping was performed to understand the distribution of the Cu-Schiff base complex on GO. The elemental mapping indicated the uniform distribution of Cu, N and Si over GO surface (Fig. 4). Further, the copper content, analyzed by AAS, was found to be 0.29 mmol g−1 in the GO–Cu. The TGA thermograms of GO and GO–Cu are displayed in Fig. S5† The TGA of GO showed ∼20% weight loss near 100 °C, due to evaporation of water molecules present in the lattice of GO. The second significant weight loss was observed in the range of 170–240 °C, due to thermal decomposition of oxygen carrying functionalities.24 The thermogram of GO–Cu showed a very small weight loss (5%) around 100 °C owing to the loss of adsorbed water molecules. The second weight loss in the temperature range from 175 to 260 °C was attributed to the thermal decomposition of undigested oxygen carrying functionalities and APTMS moieties, which have not participated in further chemical fictionalization. The last major mass loss observed in the range of 360–470 °C was related to the slow decomposition of the Cu(II) Schiff base complex.25 The BET surface area of the GO and GO–Cu, determined by nitrogen physisorption at 77 K, was about 93.599 and 42.659 m2 g−1 respectively.
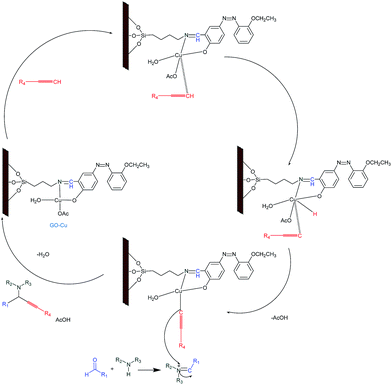
The catalytic efficiency of GO–Cu was tested in the three-component coupling of aldehydes, amines and alkynes shown in Scheme 2 (more detail of propargylamines synthesis is given in the ESI†).
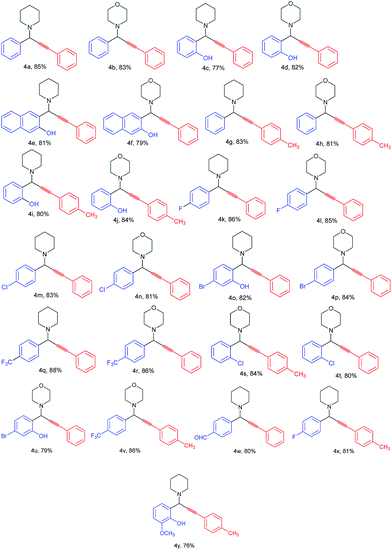
In order to optimize reaction conditions, various parameters (time, temperature, solvents and catalyst loading) were studies. Benzaldehyde, phenylacetylene and piperidine were chosen as model reactants under N2 atmosphere. These results are summarized in Table 1. When the reaction was carried out without catalyst (Table 1, entry 1) under reflux, no product was formed even after 36 h. The same reaction in presence of 20 mg of GO in water (5 mL) under reflux for 24 h yielded no product as the starting materials were recovered (Table 1, entry 2). However, when the reaction was carried out in the presence of 20 mg GO–Cu under reflux in water, the desired product was obtained in 85% yield within 8 h (Table 1, entry 3). However, no significant improvement in yield of the product was noticed when the catalyst loading was increased from 20 to 50 mg (Table 1 entry 4). It indicated that 20 mg of the catalyst was optimum. Among the various solvents used such as water, toluene, THF, DMF, neat and DMSO, water was found to be the best medium for the synthesis of propargylamines (Table 1, entries 1–12). After optimization of the reaction conditions, we choose a variety of different aldehydes, alkynes and amines to discern the catalytic scope and the generality of the GO–Cu complex (Table 2). Aryl aldehydes, possessing electron withdrawing groups, afforded better yields as compared to aldehydes bearing electron donating groups. The reaction of primary amines such as aniline and benzylamine, in place of secondary amines, with aldehydes and alkynes did not succeed to yield the expected products, thereby limiting the scope of the reaction.
| Entry | Catalyst (mg) | Time (h) | Temp. (°C) | Solvents | Yieldb, % |
|---|---|---|---|---|---|
| a Reaction conditions: benzaldehyde (1 mmol), piperidine (1 mmol), phenyl acetylene (1.2 mmol).b Isolated yield after work up. | |||||
| 1 | — | 36 | 100 | Water | — |
| 2 | GO (20) | 24 | 100 | Water | — |
| 3 | GO–Cu (20) | 08 | 100 | Water | 85 |
| 4 | GO–Cu (50) | 08 | 100 | Water | 85 |
| 5 | GO–Cu (20) | 15 | 100 | Water | 85 |
| 6 | GO–Cu (20) | 08 | 100 | — | 60 |
| 7 | GO–Cu (20) | 08 | 100 | Toluene | 84 |
| 8 | GO–Cu (20) | 08 | 110 | Toluene | 84 |
| 9 | GO–Cu (20) | 08 | 100 | THF | 62 |
| 10 | GO–Cu (20) | 08 | 70 | THF | 58 |
| 11 | GO–Cu (20) | 08 | 100 | DMF | 50 |
| 12 | GO–Cu (20) | 08 | 100 | DMSO | 45 |
The coupling proceeded smoothly with secondary amines such as morpholine and piperidine to afford the corresponding propargylamines in good yields under standard conditions, and it was found that piperidine gave better results in term of yields and reaction time than morpholine. The products were obtained as yellowish oil and fully characterized by IR and 1H NMR. Furthermore, the catalyst (GO–Cu) was compared with previously reported catalysts for the synthesis of propargylamines in terms of TON and TOF (Table 3). Our catalyst (0.58 mol%, based on Cu) gave better TON (146) and TOF (18.3) in the reaction. Furthermore, the catalyst was tested for recyclability up to six times. It is apparent from (S6†) that the GO–Cu catalyst could be reused up to four cycles without loss of catalytic activity. However, gradual decline in the catalytic activity of GO–Cu was noted after 5th cycle. A plausible mechanism for the synthesis of propargylamines using GO–Cu complex is proposed in Scheme 3. Based upon the earlier reports,2a it is suggested that the first step of the reaction involves the activation of the C–H bond of alkyne by Cu Schiff's base complex. The alkenyl–Cu Schiff's base complex intermediate thus formed reacted with the iminium ion generated in situ from aldehyde and secondary amines to give the corresponding propargylamines and regenerated the GO–Cu complex for further reaction.
Conclusions
In summary, we have developed a new Cu(II) Schiff base complex immobilized onto graphene oxide and demonstrated its catalytic application in the green synthesis of propargylamines from aldehydes, alkynes and amines without using any base or co-catalyst in aqueous medium. The developed catalyst is very cheap, easily recovered at the end of the reaction, and recycled up to four times without significant loss in catalytic activity.Acknowledgements
We are thankful to the CRF ISM, SAIF IIT Madras, SAIF Panjab University, STIC, Kochi, and IISER Bhopal for providing help in the analysis of the samples. SK acknowledges the receipt of ISM fellowship.Notes and references
- (a) R. K. Rit, M. R. Yadav, K. Ghosh, M. Shankar and A. K. Sahoo, Org. Lett., 2014, 16, 5258–5261 CrossRef CAS PubMed; (b) A. Fodor, A. Kiss, N. Debreczeni, Z. Hell and I. Gresits, Org. Biomol. Chem., 2010, 8, 4575–4581 RSC.
- (a) S. Samai, G. C. Nandi and M. S. Singh, Tetrahedron Lett., 2010, 51, 5555–5558 CrossRef CAS; (b) B. Sreedhar, P. S. Reddy, C. S. V. Krishna and P. V. Babu, Tetrahedron Lett., 2007, 48, 7882–7886 CrossRef CAS.
- (a) M. E. Jung and A. Huang, Org. Lett., 2000, 2, 2659–2661 CrossRef CAS PubMed; (b) T. Murai, Y. Mutoh, M. Ohta and M. Murakami, J. Am. Chem. Soc., 2004, 126, 5968–5969 CrossRef CAS PubMed.
- (a) V. Srinivas and M. Koketsu, Tetrahedron, 2013, 69, 8025–8033 CrossRef CAS; (b) L. Zani and C. Bolm, Chem. Commun., 2006, 4263–4275 RSC.
- (a) K. K. R. Datta, B. V. Subba Reddy, K. Ariga and A. Vinu, Angew. Chem., Int. Ed., 2010, 49, 5961–5965 CrossRef CAS PubMed; (b) D. Aguilar, M. Contel and E. P. Urriolabeitia, Chem.–Eur. J., 2010, 16, 9287–9296 CrossRef CAS PubMed; (c) A. Teimouri, A. N. Chermahini and M. Narimani, Bull. Korean Chem. Soc., 2012, 33, 1556–1560 CrossRef CAS; (d) B. J. Borah, S. J. Borah, K. Saikia and D. K. Dutta, Catal. Sci. Technol., 2014, 4, 4001–4009 RSC.
- (a) M. Jeganathan, A. Dhakshinamoorthy and K. Pitchumani, ACS Sustainable Chem. Eng., 2014, 2, 781–787 CrossRef CAS; (b) M. Kidwai, V. Bansal, A. Kumar and S. Mozumdar, Green Chem., 2007, 9, 742–745 RSC; (c) L. Pin-Hua and W. Lei, Chin. J. Chem., 2005, 23, 1076–1080 CrossRef; (d) K. Namitharan and K. Pitchumani, Eur. J. Org. Chem., 2010, 2010, 411–415 CrossRef; (e) P. Li, Y. Zhang and L. Wang, Chem.–Eur. J., 2009, 15, 2045–2049 CrossRef CAS PubMed.
- (a) X. Xiong, H. Chen and R. Zhu, Catal. Commun., 2014, 54, 94–99 CrossRef CAS; (b) V. Srinivas and M. Koketsu, Tetrahedron, 2013, 69, 8025–8033 CrossRef CAS; (c) G. Villaverde, A. Corma, M. Iglesias and F. Saanchez, ACS Catal., 2012, 2, 399–406 CrossRef CAS.
- (a) S. Kumari, A. Shekhar and D. D. Pathak, RSC Adv., 2014, 4, 61187–61192 RSC; (b) S. S. Li, J. J. Lv, Y. Y. Hu, J. N. Zheng, J. R. Chen, A. J. Wang and J. J. Feng, J. Power Sources, 2014, 247, 213–218 CrossRef CAS.
- Y. L. Min, K. Zhang, W. Zhao, F. C. Zheng, Y. C. Chen and Y. G. Zhang, Chem. Eng. J., 2012, 194, 203–210 CrossRef.
- H. P. Mungse, S. Verma, N. Kumar, B. Sain and O. P. Khatri, J. Mater. Chem., 2012, 22, 5427–5433 RSC.
- H. Su, Z. Li, Q. Huo, J. Guan and Q. Kan, RSC Adv., 2014, 4, 9990–9996 RSC.
- S. Verma, M. Aila, S. Kaul and S. L. Jain, RSC Adv., 2014, 4, 30598–30604 RSC.
- (a) S. Kumari, A. Shekhar and D. D. Pathak, Chem. Sci. Trans., 2014, 3, 45–54 Search PubMed; (b) S. Kumari, A. Shekhar and D. D. Pathak, Chem. Sci. Trans., 2014, 3, 652–663 Search PubMed.
- (a) Y. Gu, Green Chem., 2012, 14, 2091–2128 RSC; (b) E. N. Ntainjua, M. Piccinini, S. J. Freakley, J. C. Pritchard, J. K. Edwards, A. F. Carley and G. J. Hutchings, Green Chem., 2012, 14, 170–181 RSC.
- S. Kumari, A. Shekhar, H. P. Mungse, O. P. Khatri and D. D. Pathak, RSC Adv., 2014, 4, 41690–41695 RSC.
- (a) Y. Gao, Y. Zhang, C. Qiu and J. Zhao, Appl. Organomet. Chem., 2011, 25, 54–60 CrossRef CAS; (b) M. Santos, F. E. Kuhn, W. M. Xue and E. Herdtweck, Dalton Trans., 2000, 3570–3574 RSC; (c) P. K. Khatri, S. Choudhary, R. Singh, S. L. Jain and O. P. Khatri, Dalton Trans., 2014, 43, 8054–8061 RSC; (d) R. Nie, J. Wang, L. Wang, Y. Qin, P. Chen and Z. Hou, Carbon, 2012, 50, 586–596 CrossRef CAS.
- (a) M. Ozdemir, Inorg. Chim. Acta, 2014, 421, 1–9 CrossRef CAS; (b) A. Stuart, Infrared Spectroscopy: Fundamentals and Applications, J. Wiley, Chichester, West Sussex, England, Hoboken, NJ, 2004 Search PubMed.
- Anuradha, S. Kumari and D. D. Pathak, Tetrahedron Lett., 2015, 56, 4135–4142 CrossRef CAS.
- (a) K. Nakamoto, Coordination Compounds, in Infrared and Raman Spectra of Inorganic and Coordination Compounds, John Wiley and Sons, Inc., New York, 4th edn, 1986 Search PubMed; (b) Z. Li, S. Wu, H. Ding, D. Zheng, J. Hu, X. Wang, Q. Huo, J. Guan and Q. Kan, New J. Chem., 2013, 37, 1561–1568 RSC.
- Z. Li, S. Wu, H. Ding, H. Lu, J. Liu and Q. Kan, New J. Chem., 2013, 37, 4220–4229 RSC.
- (a) F. Liu, J. Sun, L. Zhu, X. Meng, C. Qi and F.-S. Xiao, J. Mater. Chem., 2012, 22, 5495–5502 RSC; (b) M. Ali Nasseri, A. Allahresani and H. Raissi, RSC Adv., 2014, 4, 26087–26093 RSC.
- (a) M. Salavati-Niasari and M. Bazarganipour, Catal. Commun., 2006, 7, 336–343 CrossRef CAS; (b) Z. Li, S. Wu, D. Zheng, H. Liu, J. Hu, H. Su, J. Sun, X. Wang, Q. Huo, J. Guan and Q. Kan, Appl. Catal., A, 2014, 470, 104–114 CrossRef CAS; (c) Y. Yang, S. Hao, Y. Zhang and Q. Kan, Solid State Sci., 2011, 13, 1938–1942 CrossRef CAS.
- (a) N. Anand, K. H. P. Reddy, K. S. R. Rao and D. R. Burri, Catal. Lett., 2011, 141, 1355–1363 CrossRef CAS; (b) C. K. P. Neeli, S. Ganji, V. S. P. Ganjala, S. R. R. Kamarajua and D. R. Burri, RSC Adv., 2014, 4, 14128–14135 RSC.
- (a) H. P. Mungse, S. Verma, N. Kumar, B. Sain and O. P. Khatri, J. Mater. Chem., 2012, 22, 5427–5433 RSC; (b) S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef CAS.
- F. Liu, J. Sun, L. Zhu, X. Meng, C. Qi and F.-S. Xiao, J. Mater. Chem., 2012, 22, 5495–5502 RSC.
- (a) L. Lili, Z. Xin, G. Jinsena and X. Chunming, Green Chem., 2012, 14, 1710–1720 RSC; (b) K. Layek, R. Chakravarti, M. L. Kantam, H. Maheswaran and A. Vinu, Green Chem., 2011, 13, 2878 RSC; (c) C. Wei and C.-J. Li, J. Am. Chem. Soc., 2003, 125, 9584–9585 CrossRef CAS PubMed; (d) X. Zhang and A. Corma, Angew. Chem., 2008, 120, 4430–4433 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and spectroscopic results of synthesized propargylamines. See DOI: 10.1039/c5ra25209a |
| This journal is © The Royal Society of Chemistry 2016 |