Fabricating binary anti-corrosion structures containing superhydrophobic surfaces and sturdy barrier layers for Al alloys
Abstract
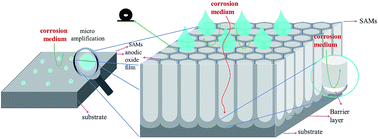
Aluminum alloys with novel binary anti-corrosion structures on the surface showing superhydrophobic properties were fabricated via chemical etching, anodic oxidation and chemical modification. Surface morphologies and chemical elements of the as-prepared films were investigated by Fourier transform infrared spectrometer, scanning electron microscopy and confocal laser scanning microscope. Surface wettability was investigated by the contact angle meter. Manipulation of surface morphology by anodic oxidation current density and the influence of surface chemical modification on the wettability were investigated. The anti-corrosion properties of the as-prepared films were characterized using an electrochemistry workstation. The results showed that surface water contact angle could reach 156° after chemical modification when the current density of anodic oxidation was 5 A dm−2. The corrosion potential (Ecorr) was positively increased from −1189 mV for bare Al alloys to −304 mV for the samples anodized at 5 A dm−2. The synergetic effect between the protective properties of air trapped in a low adhesion superhydrophobic surface and good barrier properties of the barrier layer of anodic oxidation film was remarkably enhanced the corrosion resistance of aluminum alloys. Influences of the anodic oxidation current density and the self-assembled films on the anti-corrosion performance were discussed in detail.

 Please wait while we load your content...
Please wait while we load your content...