High-performance SPEEK/amino acid salt membranes for CO2 separation
Abstract
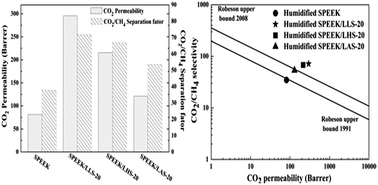
Three types of amino acid salts (sodium lysine, sodium arginine, sodium histidine) were introduced into SPEEK polymer matrix to fabricate a series of SPEEK/amino acid salt membranes for CO2 separation. The membranes could efficiently separate CO2 from a CO2/CH4 mixture due to the simultaneous improvement of CO2 permeability and separation performance. Firstly, the amino acid salt contained a larger number of amino groups (–NH2), which could reversibly react with CO2, improving the CO2 molecules transfer performance. Secondly, the ether oxygen structure (–O–) in the polymer chain and the carboxyl groups (–COO−) in the amino acid salt had an excellent affinity for CO2, enhancing the solubility selectivity. Thirdly, the introduction of amino acid salt increased the water uptake, and water was favored over CO2 dissolution; this was also good for the increase of solubility selectivity. Among the three types of amino acid salts, the membrane doped with 20 wt% sodium lysine (SPEEK/LLS-20) showed the highest CO2 permeability of 295.6 barrer and CO2/CH4 selectivity of 71.8 at 2 bar and room temperature, surpassing the 2008 Robeson upper bound line.

 Please wait while we load your content...
Please wait while we load your content...