Impact of blend ratio on the co-firing of post-methanated distillery effluent solid waste and low-rank Indian coal via analysis of oxidation kinetics through TGA
C. Naveen and
M. Premalatha*
Solar Energy Laboratory, Department of Energy & Environment (DEE), National Institute of Technology (NIT), Tiruchirappalli 620 015, Tamil Nadu, India. E-mail: latha@nitt.edu; Tel: +0431-250313244
First published on 2nd March 2016
Abstract
Distillery effluent is usually discharged in open pond systems after anaerobic digestion. Due to strict norms made by Environmental Protection Acts, alternative ways are being explored to treat the effluent further and also recover the possible energy content from the distillery effluent. In this study, an attempt has been made to study the oxidation kinetics of dried Post-Methanated Distillery Effluent (PMDE) solid waste blended with Low Rank Indian Coal (LRIC) at different blending ratios in a thermogravimetric analyzer (TGA). Heating rates of 10 °C min−1 and 100 °C min−1 were used. Due to the low reactivity of PMDE, a slight tendency of detraction in the overall reactivity of the blended samples was observed during combustion. Increase in the blending ratio of PMDE does not have much significant detraction in primary reactions of the thermal decomposition of blended samples, at both heating rates. Also for higher blending ratios, a significant improvement in the required activation energy for burning of blended samples was noted.
1. Introduction
Industrial sectors generate huge quantities of waste water, which contains considerable quantities of organic solid waste. It is a potential energy source, where huge energy recovery is possible. Distilleries are among the most water intensive industries, and have been ranked as the heaviest polluter among the other few industrial sectors in India.1 In distilleries, a major fraction (∼90%) of the fermented wash going to distillation column is discharged as wastewater named spent wash. In India, 319 distilleries are producing 3.25 billion liters of alcohol and discharge approximately 40.4 billion liters of spent wash per annum.2 Spent wash has a high BOD and COD content. Hence treating spent wash is a challenging research area, envisaging a zero discharge solution with energy recovery option. The bio-methanation process is the traditional method used for treating spent wash in high rate digesters, with significant amount of energy recovery.3 The effluent received after anaerobic treatment is called post-methanated distillery effluent (PMDE). The PMDE still contains high organic matters loading with dark color.4 It is estimated that an industry producing 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 liters per day of alcohol will approximately discharge 700
000 liters per day of alcohol will approximately discharge 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 liters of PMDE containing 65
000 liters of PMDE containing 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm of solids per liter. Hence the potential of solid waste from such an industry is 45.5 tonnes per day. This kind of organic waste from industries is named as second generation biofuels.5 Efficient ways of treating PMDE are reverse osmosis (RO) and drying of biomethanated effluent.3 Dried powder of PMDE waste will have a good calorific value which could be used as a good source of fuel for power generation.4 Concentration and combustion of effluent technique are still under testing6 and not much research work are available on this technique. The ash collected after combustion of PMDE is rich in inorganic salt content like potassium, magnesium, etc., which could also be recovered by a suitable chemical process.7 Potassium rich ash could be used as a good fertilizer in agricultural lands.6
000 ppm of solids per liter. Hence the potential of solid waste from such an industry is 45.5 tonnes per day. This kind of organic waste from industries is named as second generation biofuels.5 Efficient ways of treating PMDE are reverse osmosis (RO) and drying of biomethanated effluent.3 Dried powder of PMDE waste will have a good calorific value which could be used as a good source of fuel for power generation.4 Concentration and combustion of effluent technique are still under testing6 and not much research work are available on this technique. The ash collected after combustion of PMDE is rich in inorganic salt content like potassium, magnesium, etc., which could also be recovered by a suitable chemical process.7 Potassium rich ash could be used as a good fertilizer in agricultural lands.6
In India most of the distilleries are attached to sugar mills.8 Bagasse and molasses are produced as waste during sugar production. Molasses is used as raw material in distilleries and bagasse as a fuel in co-generation plants in sugar mills. The installed capacity of cogeneration in sugar industries is 2393 MW as on 2013 in India.9 Cogeneration power plants available at many sugar mills are exporting power to the grid throughout the year. Due to the unavailability of sugarcane during off-seasons, bagasse is not available over the year for power generation. So Low Rank Indian Coal (LRIC) (high in ash content) is majorly used for cogeneration power plants in India to meet the adequate fuel supply for year-round cogeneration. To enhance the ignition and burn-out characteristics of low rank coal it is necessary to add some supplementary fuels. Co-combustion technologies for low-rank coals with biomass are generally suitable, cost effective and well recognized technology to improve the combustion characteristics of low rank coal. Very few numbers of literatures are available on co-combustion conversion mechanisms of mixing biomass or industrial biomass waste with low rank coal.10–16 The mixing of biomass can enhance the ignition characteristics of low-rank coals during co-combustion because of the high volatile matter (VM) content in the biomass.10 When waste biomass was mixed with low rank coal, the unforeseen effects were reported on the maximum burning rates as well as the burnout level.11 Also it is reported that the mixing ratio of biomass plays a vital role on burning characteristics with combined effects of type of atmosphere used for combustion (dry air or oxygen).11 Non-synergistic behaviour was observed in burning profiles of Mukah Balingian coal with palm biomass blends, indicating that both the materials combust individually.12 With an increase in biomass blending ratios, combustion index of the low-rank coal and biomass increased sharply.13 This will avoid a percentage of coal used at the power plant and will improve the overall performance of power plant. However, adding more biomass materials with coal frequently causes serious problems in the conventional combustors.11 Hence a study of nature of thermal decomposition of PMDE solid waste with the low rank Indian coal (LRIC) is essential to design an appropriate thermal conversion system. Moreover, the simplest way of PMDE disposal is achieved effectively by pre-mixing the waste with the coal and by feeding the mixed fuel into the bunkers for burning. This co-firing approach is possible only up to 30% mixing of PMDE solid waste on a thermal basis with coal, since due to high alkaline chlorides constituents in PMDE solid waste.4 Although many technical issues are yet to be resolved, co-combustion of PMDE solid waste may be the best energy recovery option for sugar mill power producers at present.
Thus far, the study on co-combustion of low rank Indian coal (LRIC) and PMDE solid waste has not been reported. Therefore, the main purpose of this work is to characterise the thermal behaviour of PMDE solid waste with low rank Indian coal and their respective blends under oxidative atmospheres via thermo gravimetric analysis. Thermal degradation kinetics plays a key role in design, operation, and modelling of a combustion process. Thermo gravimetric analysis (TGA) has been used extensively to determine the thermal degradation characteristics and kinetic parameters of blended fuels during thermochemical conversion.17 The nature of PMDE solid waste is far different from coal. Thus, it is important to understand the individual behaviour of the coal and PMDE solid waste as well as their synergetic influence in combustion efficiency with different blending ratios at different heating rates. The co-combustion may or may not be beneficial. Therefore, the compatibility of blended fuels with respect to their combustion performance has to be properly evaluated.
2. Experimental
2.1. Materials
Post-methanated distillery effluent (PMDE) used in this study was collected from the anaerobic digestion plant of distillery at different time periods over the day and mixed well. Collected samples were kept for sun drying for two days. Low rank Indian coal (LRIC) used for this study was obtained from local sugar mill cogeneration plant. PMDE and LRIC were first sun dried for 3–5 days for moisture removal. The samples were pulverised using mortar and pestle and sieved to the desired particle size of <105 μm to ensure the uniformity of particle size distribution suitable for TGA pan.Different blending ratios of the PMDE/LRIC samples were attained by weighing each sample directly into a glass vial on a Denver semi-micro balance (±0.01 mg) and mixed in appropriate proportions, followed by vortexing each vial for ten minutes to insure a homogeneous distribution. Different mixtures of both PMDE with LRIC materials were prepared. These included 10, 20, and 30 wt% of PMDE solid waste (10PM90LC, 20PM80LC and 30PM70LC, respectively). The raw samples are named as 100LC (LRIC only) and 100 PM (PMDE solid waste only). Samples were kept in a tightly screwed cap bottles.
2.2. Energy content
The gross calorific value of PMDE solid waste and LRIC were measured using bomb calorimeter system IKA/C 5000 control in adiabatic mode. Benzoic acid tablets were used as calibration standard. For all combustion tests, the samples in pellet form were ignited in the bomb saturated with 30 bar of pure oxygen. Error due to sampling was avoided by conducting the test thrice for each sample. Average results are reported with the standard deviation from the average value. The energy content of the sun dried PMDE solid wastes was 2320.4 kcal kg−1 on wet basis, SD-2.306 and for low rank Indian coal (LRIC) is 2687.5 kcal kg−1 on wet basis, SD-1.806.2.3. Proximate analysis & ultimate analysis
PMDE/LRIC samples were characterized in terms of proximate analysis to provide information on moisture, ash, volatile matter and fixed carbon contents of the material. Proximate analysis for individual samples were also carried out using American Society for Testing and Materials (ASTM) methods (D5142-09)4 by using a Perkin Elmer TGA 4000 series.Ultimate analysis of PMDE/LRIC was performed to determine carbon, hydrogen, and nitrogen content in the samples by using PerkinElmer 2400 Series II CHNS Elemental Analyzer. Oxygen content was calculated by subtracting the ash and the C, H, N & S content of the sample. Both ultimate and proximate analyses were performed thrice. Average results are reported with the standard deviation from the average value. The results of ultimate and proximate analyses are listed in Table 1.
| Characteristics of fuels | PMDE solid waste | Low rank Indian coal | |||
|---|---|---|---|---|---|
| Content in (wt%) | SD | Content in (wt%) | SD | ||
| Proximate analysis | Moisture | 9.76 | 0.25 | 2.48 | 0.11 |
| Volatile matter | 43.15 | 2.01 | 18.5 | 0.2 | |
| Fixed carbon | 21.77 | 0.67 | 23.03 | 0.2 | |
| Ash | 24.17 | 2.02 | 56.03 | 0.35 | |
| Ultimate analysis | C | 33.75 | 1.04 | 26.32 | 0.25 |
| H | 3.20 | 0.20 | 2.65 | 0.12 | |
| N | 3.44 | 0.16 | 1.12 | 0.10 | |
| S | 1.63 | 0.17 | 0.44 | 0.03 | |
| O | 33.80 | 2.23 | 13.32 | 0.24 | |
2.4. Ash analysis
Inorganic elemental composition of ash obtained from PMDE and LRIC were found using PerkinElmer Optima 2000 DV inductively coupled plasma optical emission spectrometry (ICP-OES). Inorganic chemical composition of ash obtained from PMDE & LRIC are reported in Table 2 on wt% (weight percentage) basis.| Constituents | PMDE solid waste ash | Low rank Indian coal ash | ||
|---|---|---|---|---|
| Concentration range in (wt%) | SD | Concentration range in (wt%) | SD | |
| Silica (SiO2) | 13.8 | 0.70 | 61.8 | 0.4 |
| Aluminium oxide (Al2O3) | 0.30 | 0.06 | 24.4 | 0.2 |
| Iron oxide (Fe2O3) | 0.40 | 0.09 | 5.4 | 0.04 |
| Titanium oxide (TiO2) | Traces | — | 1.3 | 0.02 |
| Calcium oxide (CaO) | 1.9 | 0.10 | 2.1 | 0.01 |
| Magnesium oxide (MgO) | 11.7 | 0.52 | 0.6 | 0.01 |
| Potassium oxide (K2O) | 36.85 | 1.20 | 1.4 | 0.03 |
| Sulphate (SO3) | 7.2 | 0.55 | 1.2 | 0.01 |
| Sodium (Na2O) | 3.6 | 0.35 | 0.8 | 0.01 |
| Remaining are Cl and other traceable | 24.25 | 1.23 | Nil | — |
2.5. Thermal degradation analysis-TGA
The combustion characteristics of Low Rank Indian Coal (LRIC), PMDE solid waste and their blends were studied using a TGA analyzer (Perkin Elmer TGA 4000 series). The precision of temperature measurement of this unit was ±0.8 °C; sensitivity of microbalance was less than 0.1 μg and accuracy of ±0.02%, with the heating rates ranging from 1 to 100 °C min−1. In ensuring accurate results for this investigation, the furnace of the instrument was calibrated using Curie point reference materials (indium/nickel) and weight calibration was carried out by following its standard procedures mentioned in the manual. In order to create a good contact between the crucible and the sample, approximately 16 ± 1 mg of representative samples were uniformly packed onto the alumina pan. Using auto sampler arrangement the alumina crucible was placed over the sample holder (inside the furnace). The furnace temperature was set to increase from the 35 °C to 950 °C at two constant heating rates conditions of 10 °C min−1 (slower heating rate) & 100 °C min−1 (higher heating rate) respectively. 99.99% ultra-high pure oxygen were used as the purge gas at a flow rate of 20 cm3 min−1 at combustion condition. The mass of a sample in a controlled atmosphere is continuously recorded as a function of temperature buy a controlled temperature program and Pyris software was used to analyse the data.2.6. Kinetic study
Kinetic study for the PMDE solid waste, low rank Indian coal and their respective blends under oxidative atmospheres aims to quantify the parameters required to design an efficient combustor for energy recovery purposes.TG (thermogravimetric) and DTG (derivative thermogravimetric) curves of the dried PMDE solid waste, LRIC and their respective blend were analyzed at two different heating rates (10 & 100 °C min−1) at oxygen atmosphere. In the present work, study of kinetic schemes for oxidative atmospheres is based on the established using modified Arrhenius equation based technique.4,17,18 This will relate the effect of different blending ratios of fuels and heating rate on activation energy, order of reaction and frequency factor.
Kinetic parameters were calculated from the TGA data based on the rate equation generally adopted:
 | (1) |
The reaction rate constant could be experimentally obtained by the Arrhenius decomposition equation:
| k = Ae−Ea/RT | (2) |
Applying the Arrhenius eqn (2) for k in eqn (1) leads to a linear form eqn (3) as:
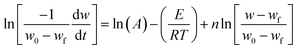 | (3) |
This eqn (3) is resembles the simplified form of equation as
| y = B + Cx + Dz, |
PYRIS software data will gives the data of time and temperature at which maximum weight loss occurred at different heating rates in oxidation atmosphere. Using this, the constants x, y and z were calculated. Constants B, C, D are estimated by multi-linear regression of the TGA data for each stage using LINEST function in Microsoft Excel. By finding out the values of constants the kinetic parameter results (pre-exponential factor, activation energy and reaction order) and the conversion for each experiment could be predicted for the studied parameters of operations.
3. Results and analysis
The samples were heated at lower heating rate at a ramp rate of 10 °C min−1 to get sharper, smoother peaks with higher peak conversion rates. The samples were also heated at a higher ramp rate of 100 °C min−1 up to 950 °C to mimic fast oxidation to observe the real time study happening in real pulverized coal combustion system. The mass loss conversion profiles, kinetic parameters of the LRIC with PMDE solid waste blends at lower and higher heating rates are analyzed. Mass loss conversion profiles of different blends were compared for three important characteristic parameters of ignition, peak and final or burnout temperatures using thermograph. Ignition temperature (Tignition °C) is the temperature, at which sudden decrease in weight loss is observed on the DTG curve. This will determine the minimum temperature, at which a fuel will undergo spontaneous combustion. Peak temperature (Tp °C) is the temperature at which maximum rate of combustion (DTGmax) occurs on DTG curve. Peak temperature and its corresponding rate is a measure of combustibility and reactivity respectively. Low peak temperature indicates easier ignition of fuel. Burnout temperature (Tb °C) is the temperature at which no further mass losses is observed on the DTG curve.3.1. Thermal degradation of dried PMDE solid waste, LRIC and their respective blends at lower heating rate on oxidative atmospheres
The TG and DTG curves represents the conversion rates of dried PMDE solid waste, LRIC and their respective blends at oxidation mode for a heating rate of 10 °C min−1 are shown in the Fig. 1 & 2 respectively.Three distinct stages of decomposition occurred when raw PMDE solid waste (100 PM) was heated under oxidative atmosphere at 10 °C min−1. The first small peak (P1) occurred between 35–135 °C due to moisture evaporation. The second peak (P2) occurred between 207.12–357.86 °C due to de-volatilization within the material. Third peak (P3) occurred between 527.16–627.92 °C, this could be due to oxidation of char material. P3 has maximum rate of combustion (DTGmax) was around −10.12% min−1. Similar observations were reported for thermal decomposition of grape pomace,19 tomato plant20 and Spirulina wastes21 at oxidative atmospheres. A small mass loss (%) was observed after P3 peak. This is mainly due to the decomposition of complicated carbonaceous materials retained in the PMDE solid waste which burns completely at higher temperature range or by keeping the sample at isothermal condition for more resident time at 950 °C.4
Two distinct stages of decomposition occurred when raw LRIC (100 LC) were heated under oxidative atmosphere at 10 °C min−1. The first small peak (P1) occurred between 35–135 °C and is due to moisture evaporation. The second peak (P2) occurred between 270.09–600.07 °C and is due to the simultaneous de-volatilization and char oxidation within the material11,22,23 and has maximum rate of combustion (DTGmax) as −4.142% min−1.
Three distinct stages of decomposition occurred for the blended samples of LRIC and PMDE (10PM90LC, 20PM80LC and 30PM70LC) when heated under oxidative atmosphere at 10 °C min−1. For all the blends the first small peak (P1) occurred between 35–135 °C due to moisture evaporation. The second peak (P2) appeared in the range of 207–570 °C showing the de-volatilization and char oxidation within the material. The P2 weight loss is mainly contributed by LRIC. The third small peak (P3) occurred adjacent to the second peak between 540–640 °C, mainly due to char oxidation of blended PMDE waste.
The ignition temperature (Tignition) of blended samples got reduced to lower temperature (207.12 °C) when compared to the ignition temperature of 100LC. LRIC blended with PMDE. This is because 100 LC start releasing volatiles at 270.09 °C and 100 PM was releasing volatile matter early at a lower temperature of (207.12 °C). So time and temperature needed for combustion of the blends was advanced due to the emission and combustion of volatile from the PMDE waste which causes it to burn at a lower temperature range. Same scenario was noted for co-combustion of coal/biomass blends24 and municipal solid waste with high ash Indian coal.22
Fig. 2 shows, with respect to increase in blending ratio of PMDE to LRIC the height of second peak (P2) decreases and third peak (P3) increases proportionately. P2 & P3 curves represent the behavior of sum of individual components in the blend, which suggests that the blending process does not affect the combustion behavior of the individual components. From Table 3, the maximum rate of combustion (DTGmax) of P2 curve of blended samples decreased considerably from −4.142% min−1 to ∼−3.00% min−1 when compared to 100 LC, due to slow combustible nature of blended PMDE char in the P2 temperature range. Also it is interesting to note that in case of blending of PMDE, there is a common tendency of slight shifting of peak temperature (Tp °C) of P2 & P3 towards right side (486.84 to ∼493 °C) & (547.54 to ∼568 °C) for blended samples when compared to the 100 LC sample. It shows that although interactions between the two components of the blend are not so significant, interactions slightly tend to affect the reactivity of the LRIC in the blends. The maximum rate of combustion (DTGmax) and peak temperature (Tp °C) of P2 curve was almost same with the increase of PMDE addition to the LRIC. Hence higher blending ratio of PMDE didn't affect much in the combustion efficiency of the LRIC present in the blend, since P2 is mainly due to LRIC. But higher blending ratio enhanced the combustion efficiency of PMDE present in the blend, since P3 is mainly due to PMDE. For higher bending ratio the maximum rate of combustion (DTGmax) increased and peak temperature (Tp °C) of P3 curve moved left hand side shows that interactions between the two blend components are significant, interactions slightly tend to enhance the reactivity of PMDE in the blends. Table 3 indicates that the residue percentage of the fuel decreases with the increase of the PMDE mixture ratio, due to the low ash content of PMDE solid waste. Calculated burnt out weight percentage was arrived by sum of two individual weight loss (from final residual weight of 100 PM and 100LRIC) with respect to its blending ratios. Complete combustion of PMDE waste is only possible at higher temperature range up to 1200 °C or by keeping the sample at isothermal condition for more resident time (30 minutes) at 950 °C due to more complex nature of fixed carbon.4 Almost approximately 6% of carbon of PMDE waste will be unburnt due to its complex nature. So we checked the effect of blending ratio in final burnt percentage of blend by comparing with calculated final burnt out weight percentage. Table 4 shows that calculated burnt out weight percentage of blended samples are almost same as experimental value, implies that there was not much significant interactions between the blends to burn more complex carbon matters present in the PMDE waste.
| Sample | P2 | P3 | Tf (°C) | Residue (wt%) | ||||
|---|---|---|---|---|---|---|---|---|
| Temperature range (°C) | Tp (°C) | DTGmax (% min−1) | Temperature range (°C) | Tp (°C) | DTGmax (% min−1) | |||
| 100 PM | 207.12–367.89 | 291.44 | −0.873 | 527.16–627.92 | 547.54 | −10.129 | 627.59 | 31.38 |
| 100 LC | 270.09–600.07 | 486.84 | −4.142 | — | — | — | 600.07 | 56.97 |
| 10PM90LC | 207.12–556.75 | 491.77 | −3.126 | 556.75–637.92 | 568.56 | −0.866 | 637.92 | 55.83 |
| 20PM80LC | 207.12–546.27 | 491.93 | −3.061 | 546.27–637.75 | 568.47 | −1.585 | 637.75 | 52.20 |
| 30PM70LC | 207.59–537.37 | 492.81 | −3.062 | 537.37–637.59 | 556.99 | −2.237 | 637.59 | 47.46 |
| Sample | Calculated burnt out weight percentage (%) | Experiential burnt out weight percentage (%) |
|---|---|---|
| 10PM90LC | 45.58 | 45.17 |
| 20PM80LC | 48.14 | 48.01 |
| 30PM70LC | 50.69 | 52.92 |
3.2. Thermal degradation of dried PMDE solid waste, LRIC and their respective blends at higher heating rate on oxidative atmospheres
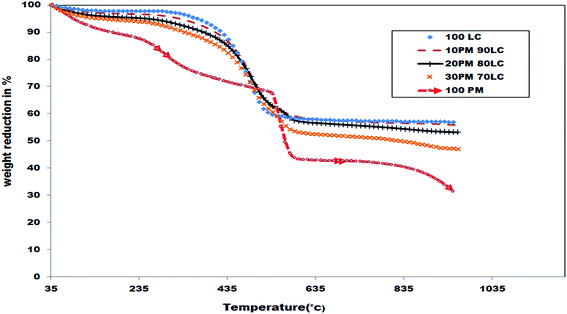
Fig. 3 & 4 shows the TG and DTG curves which represent the conversion rates of dried PMDE solid waste, LRIC and their respective blends at oxidation mode for a heating rate of 100 °C min−1.Four distinct stages of decomposition occurred when raw PMDE solid waste (100 PM) were heated under oxidative atmosphere at 100 °C min−1. The first small peak (P1) occurred between 35–135 °C and is due to moisture evaporation. The second peak (P2) occurred between 215.08–351.5 °C due to de-volatilization within the material, third peak (P3) occurred between 489.44–559.49 °C due to the oxidation of char material. The peak (P4), occurred between 783.67–871.90 °C which could be due to the oxidation of complicated char present in the PMDE material. The maximum rate of combustion (DTGmax) was around 8.138% min−1. Similarly two oxidation peaks for oxidization of biomass at 100 °C min−1 were observed in the study of co-combustion of torrefied biomass with coal.25 But at lower heating rate (10 °C min−1), the maximum char got burnt at P3 itself at lower temperature range of (547.54–627.92 °C). However final residual weight attained was same for lower and higher heating rates. This delay in char combustion (P4) at higher heating rate may be due to very less residence time.
Two distinct stages of decomposition occurred when raw LRIC (100 LC) were heated under oxidative atmosphere at 100 °C min−1. The first small peak (P1) appears around 35–135 °C and is due to moisture evaporation. The second peak (P2) occurred between 270.09–703.07 °C and is due to the overlapping of de-volatilization and char oxidation was observed in a wide band and has maximum rate of combustion (DTGmax) as −17.628% min−1. Similar kind of overlapping peaks were observed in oxidation of Venezuelan coal25 and oxidation of sub-bituminous coal.26
Two distinct stages of decomposition occurred when PMDE & LRIC blends (10PM90LC, 20PM80LC and 30PM70LC) were heated under oxidative atmosphere at 100 °C min−1. First small peaks (P1) occurred between 35–135 °C due to moisture evaporation which is common for all the three blends. The second peak occurred in the range of 207–727.82 °C. Due to very high heating rate, a wide band of decomposition occured as a result of the overlapping of at least two fractions of weight loss. First fraction (F1) of weight loss occurred in the range of 207–357.52 °C, mainly due to de-volatilization of PMDE waste. From Fig. 4 we can observe that when blending ratio of PMDE increases the weight loss in the region of F1 also increases and it exactly resembles the P2 occurred in oxidation of 100 PM at 100 °C min−1. Second fraction F2 of weight loss appeared in the range of 357.52–727.82 °C is majorly due to de-volatilization and char oxidation of both LRIC and PMDE. The P4 weight loss region of 100 PM at 100 °C min−1 was shifted to a lower temperature when it was blended with LRIC this shows a good sign for blending.
From Table 5, the maximum rate of combustion (DTGmax) of P2 curve of blended samples decreased considerably from −17.628% min−1 to −14.677% min−1 when compared to 100 LC, similar behavior was observed at a lower heating rate too. Also as similar to lower heating rate, blending of PMDE at higher heating rate resulted in a common tendency of slight shifting of peak temperature (Tp °C) of P2 towards right side (517.24 to ∼522 °C) when compared to the 100 LC sample. It shows that although heating rate was increased, interactions between the two components of the blend are similar as in lower heating rate. The percentage variation in the blend and the heating rates don't have a significant effect on the maximum rate of combustion or peak temperature shift of P2 curve. Hence higher blending ratio of PMDE didn't affect much in the combustion efficiency of the LRIC present in the blend at higher heating rate too.
| Sample | P2 | P3 & P4 | Tf (°C) | Residue (wt%) | ||||
|---|---|---|---|---|---|---|---|---|
| Temperature range (°C) | Tp (°C) | DTGmax (% min−1) | Temperature range (°C) | Tp (°C) | DTGmax (% min−1) | |||
| 100 PM | 197.12–367.87 | 273.59 | −9.226 | 457.87–559.49 | 527.48 | −7.976 | 871.90 | 32.18 |
| 783.67–871.90 | 823.56 | −8.138 | ||||||
| 100 LC | 277.73–702.01 | 517.24 | −17.628 | — | — | — | 702.01 | 56.09 |
| 10PM90LC | 223.82–710.82 | 522.85 | −15.628 | — | — | — | 710.82 | 52.98 |
| 20PM80LC | 222.77–712.91 | 522.36 | −15.024 | — | — | — | 712.91 | 51.49 |
| 30PM70LC | 222.82–715.69 | 521.79 | −14.677 | — | — | — | 715.69 | 46.43 |
The residence time given for the sample is much lower at higher heating rate when compare to lower heating rate. So the heat transferred from the furnace inside the TGA instrument to the biomass sample at higher heating rate is limited due to poor heat conducting property of biomass.27,28 This will reduce the maximum combustion possibility of biomass which lead to higher residual weight. But the residual weight percentage of all the samples are almost same, this shows that the effect of less residence time on maximum combustion possibility of the sample is negligible. Hence PMDE waste is better heat conductor than biomass at higher heating rates.
3.3. Determination of kinetic parameters
Only one major mass loss regime was observed for LRIC and PMDE + LRIC blends in the oxidation mode. Because of single step nature of thermal decomposition reaction of samples, we consider only the kinetics for the reaction zone P2. The kinetic parameters such as pre-exponential factor, activation energy, order of reaction for dried PMDE solid waste, LRIC and their respective blends at lower and higher heating rate were determined for maximum weight loss zone P2 using linear regression analysis using LINEST function in Microsoft Excel. Kinetic parameters for different heating rates and blending ratios are shown in Table 6.| Sample | Calculated burnt out weight percentage (%) | Experiential burnt out weight percentage (%) |
|---|---|---|
| 10PM90LC | 46.00 | 47.01 |
| 20PM80LC | 48.12 | 48.50 |
| 30PM70LC | 50.23 | 53.57 |
For all the samples the correlation coefficient (R2) values were greater than 0.80 for multiple-regression using LINEST function in Microsoft Excel for maximum weight loss region. So the kinetic parameters obtained are more reliable to predict the peak weight loss at different conditions. This will be more helpful to design a real-time PMDE solid waste co-firing boiler system (Table 7).
| Heating rate °C min−1 | Sample | Temp range/°C | E (kJ mol−1) | A (min−1) | n | R2 |
|---|---|---|---|---|---|---|
| 10 °C min−1 | 100 PM | 207.12–357.86 | 38.835 | 2.626 × 109 | 0.440 | 0.834 |
| 100 LC | 270.09–600.07 | 81.956 | 8.629 × 1010 | 0.982 | 0.909 | |
| 10PM90LC | 217.01–647.94 | 55.151 | 4.094 × 109 | 0.872 | 0.935 | |
| 20PM80LC | 217.01–507.95 | 45.141 | 6.849 × 108 | 0.774 | 0.906 | |
| 30PM70LC | 217.01–647.93 | 39.706 | 2.618 × 108 | 0.720 | 0.890 | |
| 100 °C min−1 | 100 PM | 188.84–361.60 | 32.930 | 1.525 × 109 | 0.531 | 0.819 |
| 100 LC | 238.74–738.14 | 49.655 | 8.659 × 109 | 0.897 | 0.976 | |
| 10PM90LC | 208.41–728.32 | 33.783 | 5.409 × 108 | 0.576 | 0.976 | |
| 20PM80LC | 208.41–728.32 | 28.073 | 2.273 × 108 | 0.558 | 0.933 | |
| 30PM70LC | 209.16–727.43 | 21.308 | 6.299 × 107 | 0.394 | 0.913 |
At both the lower and higher heating rates the activation energy required was found to decrease sharply (81.956 kJ mol−1 to 39.706 kJ mol−1) & (49.655 kJ mol−1 to 39.706 kJ mol−1) respectively, when blending ratio of PMDE solid waste increased. This shows a positive synergistic interaction between LRIC and PMDE, since the activation energy required for combustion was lowered. Therefore positive influence of blending of PMDE solid waste with LRIC is very much evident here. The same scenario is noted when biomass is blended with coal.11,23–25
At higher heating rate 100 °C min−1, the activation energy required drastically decreases from 81.956 kJ mol−1 to 49.655 kJ mol−1 for 100LC compared to a lower heating rate 10 °C min−1. However, heating rate does not have a significant improvement in activation energy value of 100 PM (38.835 kJ mol−1 to 32.930 kJ mol−1).
The pre-exponential factors (A) and order of reaction determined was also decreasing when blending ratio of PMDE solid waste increased.
4. Conclusions and recommendations
The findings of this study indicated that the blending of PMDE with LRIC provides an attractive and alternative way of PMDE waste disposal method. Due to PMDE blending, a slight tendency of detraction in the reactivity of the LRIC was observed during combustion (10 °C min−1: −4.142% min−1 to −3.062% min−1) & (100 °C min−1: −17.628% min−1 to −14.677% min−1). But at both the heating rates, at higher blending ratio of PMDE, the overall activation energy required got drastically reduced from (10 °C min−1: 81.956 kJ mol−1 to 39.706 kJ mol−1) & (100 °C min−1: 49.655 kJ mol−1 to 39.706 kJ mol−1). However, 20PM80LC sample appears to be the optimum fuel with the optimum value of activation energy and reaction rate. This revealed that blending of PMDE with LRIC has a promising potential for improving the combustion behaviour of LRIC and best energy recovery option.Acknowledgements
We acknowledge Dr A. Lawrence, BHEL-Trichy for ICPOES measurements.References
- K. V. Padoley, V. K. Saharan, S. N. Mudliar, R. A. Pandey and A. B. Pandit, J. Hazard. Mater., 2012, 219–220, 69–74 CrossRef CAS PubMed.
- D. Pant and A. Adholeya, Bioresour. Technol., 2007, 98, 2321–2334 CrossRef CAS PubMed.
- P. K. Tewari, V. S. Batra and M. Balakrishnan, Resour., Conserv. Recycl., 2007, 52, 351–367 CrossRef.
- C. Naveen and M. Premalatha, Bioresour. Technol., 2014, 174, 126–133 CrossRef CAS PubMed.
- Y. F. Huang, W. H. Kuan, P. T. Chiueh and S. L. Lo, Bioresour. Technol., 2011, 102, 9241–9246 CrossRef CAS PubMed.
- N. K. Saha, M. Balakrishnan and V. S. Batra, Resour., Conserv. Recycl., 2005, 43, 163–174 CrossRef.
- Y. Satyawali and M. Balakrishnan, J. Environ. Manage., 2008, 86, 481–497 CrossRef CAS PubMed.
- Confederation of Indian Industry, 2013.
- M. K. Mishra, N. Khare and A. B. Agrawal, IOSR Journal of Mechanical and Civil Engineering, 2014, 11, 69–78 CrossRef.
- A. Gani, K. Morishita, K. Nishikawa and I. Naruse, Energy Fuels, 2005, 1652–1659 CrossRef CAS.
- H. Haykiri-Acma, S. Yaman and S. Kucukbayrak, Appl. Therm. Eng., 2013, 50, 251–259 CrossRef CAS.
- S. S. Idris, N. A. Rahman and K. Ismail, Bioresour. Technol., 2012, 123, 581–591 CrossRef CAS PubMed.
- C. Moon, Y. Sung, S. Ahn, T. Kim, G. Choi and D. Kim, Exp. Therm. Fluid Sci., 2013, 47, 232–240 CrossRef CAS.
- H. Wu, A. J. Pedersen, P. Glarborg, F. J. Frandsen, K. Dam-Johansen and B. Sander, Proc. Combust. Inst., 2011, 33, 2845–2852 CrossRef CAS.
- M. E. Sanchez, M. Otero, X. Gómez and A. Morán, Renewable Energy, 2009, 34, 1622–1627 CrossRef CAS.
- M. Varol, A. T. Atimtay, B. Bay and H. Olgun, Thermochim. Acta, 2010, 510, 195–201 CrossRef CAS.
- A. E. Mansaray and K. Ghaly, Energy Sources, 1999, 21, 773–784 CrossRef.
- Y. El may, M. Jeguirim, S. Dorge, G. Trouvé and R. Said, Energy, 2012, 44, 702–709 CrossRef CAS.
- T. Miranda, S. Román, I. Montero, S. Nogales-Delgado, J. I. Arranz, C. V. Rojas and J. F. González, Fuel Process. Technol., 2012, 103, 160–165 CrossRef CAS.
- R. Font, J. Moltó, A. Gálvez and M. D. Rey, J. Anal. Appl. Pyrolysis, 2009, 85, 268–275 CrossRef CAS.
- L. Li, N. Zhao, X. Fu, M. Shao and S. Qin, Bioresour. Technol., 2013, 140, 152–157 CrossRef CAS PubMed.
- M. Muthuraman, T. Namioka and K. Yoshikawa, Fuel Process. Technol., 2010, 91, 550–558 CrossRef CAS.
- S. G. Sahu, P. Sarkar, N. Chakraborty and A. K. Adak, Fuel Process. Technol., 2010, 91, 369–378 CrossRef CAS.
- M. V Gil, D. Casal, C. Pevida, J. J. Pis and F. Rubiera, Bioresour. Technol., 2010, 101, 5601–5608 CrossRef PubMed.
- J. L. Goldfarb and C. Liu, Bioresour. Technol., 2013, 149, 208–215 CrossRef CAS PubMed.
- S. S. Idris, N. A. Rahman, K. Ismail, A. B. Alias, Z. A. Rashid and M. J. Aris, Bioresour. Technol., 2010, 101, 4584–4592 CrossRef CAS PubMed.
- S. Ceylan and Y. Topçu, Bioresour. Technol., 2014, 156, 182–188 CrossRef CAS PubMed.
- C. Gai, Y. Zhang, W.-T. Chen, P. Zhang and Y. Dong, Bioresour. Technol., 2013, 150, 139–148 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |