Vapour phase nucleation of ZnO nanowires on GaN: growth habit, interface study and optical properties†
Abstract
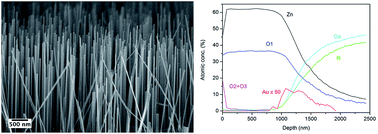
In the current work aligned ZnO nanowires were grown on p-GaN thin films for optoelectronic applications, using a vapour phase technique in a tubular furnace. To investigate the growth of ZnO nanowires at the interface with GaN, the heterojunction were characterized by scanning electron microscopy and X-ray photoelectron spectroscopy. Experimental evidence indicates that the Au catalyst remains at the interface between ZnO and GaN, and that interdiffusion of GaN into ZnO occurs. Concerning the ZnO growth, it starts with Vapour Liquid Solid (VLS) growth from Au catalyzer nanoparticles, then lateral growth takes place making nanowalls. After this initial stage, the nanowires both continue growth by VLS and start growing via a Vapour–Solid (VS) mechanism from the nanowalls. To investigate the potential of the heterostructure of ZnO nanowires on GaN as a light emitting diode, the device was also analysed by current–voltage characterization, photoluminescence and electroluminescence spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...