Amphiphobic nanocellulose-modified paper: fabrication and evaluation†
Patchiya Phanthonga,
Guoqing Guan*ab,
Surachai Karnjanakoma,
Xiaogang Haoc,
Zhongde Wangc,
Katsuki Kusakabed and
Abuliti Abudulaab
aGraduate School of Science and Technology, Hirosaki University, 1-Bunkyocho, Hirosaki 036-8560, Japan
bNorth Japan Research Institute for Sustainable Energy (NJRISE), Hirosaki University, 2-1-3, Matsubara, Aomori 030-0813, Japan. E-mail: guan@hirosaki-u.ac.jp; Fax: +81-17-735-5411; Tel: +81-17-762-7756
cDepartment of Chemical Engineering, Taiyuan University of Technology, Taiyuan 030024, China
dDepartment of Nanoscience, Sojo University, 4-22-1 Ikeda, Nishi-ku, Kumamoto 860-0082, Japan
First published on 25th January 2016
Abstract
Amphiphobic nanocellulose-modified paper with high durability is successfully fabricated using a facile two-step method. Firstly, nanocellulose-modified paper is prepared through dipping filter paper, i.e., glass microfiber (GM) filter paper and polytetrafluoroethylene (PTFE) filter paper in a dilute nanocellulose dispersed solution. Subsequently, the nanocellulose-coated paper is treated with trichloro(1H,1H,2H,2H-tridecafluoro-n-octyl)silane (FOTS) via chemical vapor deposition. The obtained paper is found to have superhydrophobicity and oleophobicity, repelling both polar and non-polar liquids, on which the drops of water and non-polar liquids with high molecular weight become marble shaped, and the contact angles of water and n-hexadecane reach 156° and 144°, respectively. Furthermore, such amphiphobic nanocellulose-modified papers exhibit excellent surface durability in several environments including at various temperatures, and in acid and alkaline solutions, salt solutions and seawater. In addition, such amphiphobic nanocellulose-modified papers show good repellant properties for several kinds of liquids from our daily life. With outstanding protection to a diverse range of liquids, the amphiphobic nanocellulose-modified paper can be applied in the fields of self-cleaning, anti-bacterial, and anti-corrosion materials.
1 Introduction
In nature, lotus leaves show self-cleaning properties, on which water drops can be removed quickly. This phenomenon inspired us to fabricate similar materials with water repellent properties. A simple and quantitative indicator to evaluate the tendency of the repelling or wetting properties of a liquid is the contact angle of the liquid on the solid surface. A hydrophilic solid surface is a surface wetted from water spreading without the formation of any droplets or the surface with a water contact angle of less than 90°; in contrast, a hydrophobic solid surface repels the spreading of water generally and has a water contact angle higher than 90°. Recently, it has been most popular to develop a solid surface with superhydrophobicity, on which water apparently forms a droplet, easily slides off, and the formed water droplet has a contact angle of larger than 150°.1–7 On the contrary, a solid surface with oil repellent properties is named an oleophobic surface. It is expected that materials can be developed with an amphiphobic surface, on which both water and oil can be repelled quickly. In other words, an amphiphobic surface combines hydrophobicity and oleophobicity, resulting in a surface with super anti-wetting properties. This anti-wetting property relates to various advantages such as being self-cleaning, anti-bacterial, anti-reflective, corrosion resistant and so on.8,9 However, the development of such kinds of materials is full of more challenges since the lower surface tension of oil generally leads to a higher solid surface attraction and as a result oil can easily wet a superhydrophobic surface.8–12 To fabricate artificial superhydrophobic surfaces, two important factors, i.e., roughness and surface energy, need to be considered. For the surface roughness, Cassie et al.13 addressed the wetting theory modelling the wettability of a rough surface. At the small protrusions of a rough surface, it cannot be filled by liquid but can be filled by air, thus only the top areas of a rough surface are wetted by liquid.1,5,6 For the surface energy, liquids with lower surface tension than the critical surface tension of the substrate will wet the surface.2 Generally, superhydrophobic surfaces are extremely low surface energy materials, especially lower than the surface tension of water (72.1 mN m−1),14 thus it will not be wetted by water. However, to fabricate superoleophobic surfaces, other factors which can protect from oil penetrating the texture should be considered.8,10–12 In addition, the lower surface tension of oil, i.e., n-hexadecane (27.47 mN m−1),14 than water is another challenge for developing the low surface tension of superoleophobic surfaces. To fabricate superamphiphobic surfaces, a specific combination of low surface energy and reentrant surface structure is needed.10–12 Li et al.11 developed a method to design and create cellulose-based natural materials with superamphiphobic properties by combining the control of cellulose fiber size and structure using plasma etching and fluoropolymer deposition. The obtained handsheets exhibited contact angles of greater than 150° for water, ethylene glycol, motor oil and n-hexadecane. Jin et al.15 also prepared amphiphobic cellulose-based materials using liquid treatments to generate the necessary roughness, followed by self-assembling a 1H,1H,2H,2H-perfluorooctyl trimethoxysilane (PFOTMS) monolayer onto the surface.Nanocellulose has recently gained great attention from researchers and industry because it has some unique properties including high tensile modulus, high specific surface area, biodegradability, biocompatibility and sustainability.16,17 Especially, nanocellulose has nanoscale dimensions and is rich in hydroxyl groups with good affinity to a variety of materials. Thus, it can be applied to make high quality paper with special surface properties or modify other solid surfaces.18 Using nanocellulose to increase the roughness and reactivity of surfaces is an attractive idea to modify substrates for achieving amphiphobicity using a natural source. Meanwhile, most research has used hard particles such as SiO2 particles,19,20 perfluoropolyether (PFPE),21 and Al2O3 nanoparticles22 for surface modification to achieve superhydrophobicity and oleophobicity.
To decrease the surface tension of substrates, silane is one kind of chemical which interacts with substrates and achieves amphiphobicity. Silane is a silicon chemical consisting of a hydrolytic center which can react with hydroxyl groups and the long tail of organic substituents.2 Many kinds of silanes have been used for the generation of amphiphobic surfaces. Jin et al.23 studied a superamphiphobic aerogel formed through the chemical vapour deposition of a membrane with various kinds of silane, in which 200 μL of (tridecafluoro-1,1,2,2-tetrahydrooctyl) trichlorosilane was used to generate amphiphobicity. Gonçalves et al.19 studied superhydrophobic cellulose nanocomposites using two kinds of fluorosiloxanes. 500 μL of 1H,1H,2H,2H-perfluorooctyl triethoxysilane was applied and obtained a water contact angle of 146.8° on their modified cellulose fiber surface. Even silane is an active chemical for producing amphiphobic surfaces, although the environmental issues of using it are a concern. Decreasing the amount of silane used in the modification will be a selective way to reduce the harmfulness to nature.
In this study, to obtain amphiphobic papers with high durability, nanocellulose was used to modify two kinds of filter papers, i.e., glass microfiber (GM) filter paper and hydrophilic polytetrafluoroethylene (PTFE) filter paper, using a dip coating method at first and then, the nanocellulose-modified paper was treated via chemical vapor deposition with 50 μL of trichloro(1H,1H,2H,2H-tridecafluoro-n-octyl)silane (FOTS). The as-obtained amphiphobic paper was characterized using a contact angle meter, scanning electron microscopy (SEM), and Fourier transform infrared spectroscopy (FTIR) for investigation of the wettability, morphology, and chemical structure of the amphiphobic surface. The durability of the amphiphobic nanocellulose-modified paper was also tested in various environments including at various temperatures, and in acid and alkaline solutions, salt solutions and seawater. In addition, 10 kinds of liquids from our daily life were used to test its repellant properties. It is expected that this will become a facile method to fabricate amphiphobic papers with high durability.
2 Experimental
2.1 Materials
Nanocellulose from bleached hardwood was provided by Daio Paper Corporation. GM filter paper (pore size: 1.6 μm; GF/A, Whatman) and hydrophilic PTFE filter paper (pore size: 0.1 μm; H010A047A, Advantec) were used as substrates. FOTS (98%, TCI, Japan) was used as received. Sulfuric acid aqueous solution (47 wt%), sodium hydroxide, sodium chloride, n-hexane, n-octane, n-hexadecane, toluene, ethylene glycol, and formamide were purchased from Wako Pure Chemical Ltd. and used without any further purification. Vacuum pump oil (ultragrade 15) was purchased from Edwards Ltd. Seawater was collected from Aomori Bay, Aomori City, Japan, and also used for testing without any pretreatment.2.2 Preparation of nanocellulose-modified substrates
The substrates (GM filter paper and hydrophilic PTFE filter paper) were cleaned by soaking them in ethanol for 5 h followed by drying them at 110 °C for 12 h. Various concentrations (0.01, 0.1 and 0.5 wt%) of nanocellulose dispersions in distilled water were prepared at room temperature. A piece of cleaned substrate was dipped into the well-dispersed nanocellulose aqueous solution for 5 h at room temperature. Then, the substrate was taken out from the dispersion solution and dried overnight at 50 °C under vacuum. As such, the nanocellulose-modified papers were obtained.2.3 Amphiphobic treatment
A piece of nanocellulose-modified paper was further treated with 50 μL of FOTS in a 25 mL bottle, which was sealed with a cap and placed in an oven at 90 °C for various periods (1–9 h). Then, the FOTS-treated paper was stored under vacuum at 50 °C overnight for the removal of the unreacted chemical.2.4 Characterization
Contact angle measurements were carried out using a contact angle meter (DMe-201, Kyowa Interface Science). A 5 μL drop of liquid (water, n-hexane, n-octane, or n-hexadecane) was applied on the surface of the substrates. The contact angle was analyzed using FAMAS software version 3.5.0. All surface contact angle values reported here were the average values of at least three measurements made on different positions of the sample surface.The surface morphology of the paper was examined using scanning electron microscopy (SEM, SU8010, Hitachi) at an acceleration voltage of 1.0 kV. A small piece of paper was fixed on carbon tape. Then, the sample was sputter-coated with Pt at 15 mA for 20 seconds to avoid charging.
The chemical composition of the substrate was characterized using Fourier transform infrared spectroscopy (FTIR) which was recorded by using a Jasco FT/IR-4200 infrared spectrophotometer with wavelengths in the range of 500–4000 cm−1. A small piece of the sample was cut and placed between two mini-KBr plates, following by pressing into a thin pellet for characterization.
2.5 Durability testing
To test the effects of various environments on the amphiphobic properties of FOTS-treated nanocellulose-modified paper the selected papers were firstly placed at different environmental temperatures (−30, 9, 30, and 50 °C) for 6 h; various concentrations of NaCl solutions (1, 3, and 5 wt%) for 6 h at room temperature, various solutions with different pH values (1, 3, 5, 7, 9, 11, and 14; prepared using 47 wt% sulfuric acid and sodium hydroxide) for 6 h at room temperature, and seawater in different periods (6, 12, and 24 h) at room temperature, respectively. Then, the pretreated papers were dried at 50 °C overnight under vacuum for the contact angle measurement.The FOTS-treated nanocellulose-modified papers were also tested by dropping 5 μL of ten different kinds of liquids directly for measuring their contact angles. These ten different liquids included seawater, sodium chloride solution (5 wt%), distilled water (7 °C), distilled water (50 °C), sulfuric acid solution (pH = 1), sodium hydroxide solution (pH = 14), toluene, ethylene glycol, formamide, and vacuum pump oil.
3 Results and discussion
3.1 Wettability
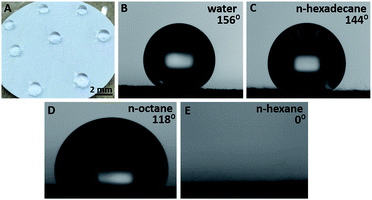
Table 1 demonstrates the contact angles of water, n-hexane, n-octane, and n-hexadecane on the FOTS-treated nanocellulose-modified GM filter papers with various loading amounts of nanocellulose and various FOTS treatment periods, compared with the untreated samples. One can see that the GM filters without nanocellulose modification and FOTS treatment can be wet by water and the other three types of non-polar liquids. This is because the GM filter paper is made of borosilicate with a fine capillary structure, which can absorb water and other liquids in fast flow rates for enabling its filtration quality. Moreover, the surface energy of unmodified GM filters should be higher than the surface tension of water and the other three types of non-polar liquids. For the nanocellulose-modified GM filter papers with various concentrations of nanocellulose but without FOTS treatment, they also show good affinity to water and the three non-polar liquids since the loaded nanocellulose also has a number of hydroxyl groups, which can create hydrogen bonds with water and organic molecules, making the liquids spread over the surface.1,2 In contrast, for the FOTS-treated papers, the wettability is at the level of hydrophobicity and oleophobicity. Interestingly, for the 0.1 wt% nanocellulose-modified GM filter paper, after it is treated with FOTS for 5 h, its surface exhibits superhydrophobicity with a water contact angle as large as 156° and a water droplet on the surface becomes a marble (Fig. 1A and B). In addition, the nanocellulose concentration has an effect on achieving amphiphobicity. Furthermore, its surface also repels the non-polar liquids with high molecular weights, i.e. n-hexadecane and n-octane, and the contact angles reach 144° and 118°, respectively (Fig. 1C and D). It indicates that the obtained surface has both superhydrophobicity and near superoleophobicity in this case. However, it should be noted that it has no oleophobicity for the non-polar liquids with a low molecular weight such as n-hexane (Fig. 1E). The different wettability between the low and high molecular weight non-polar liquids comes from the distinctive liquid surface tensions at 20 °C of n-hexane, n-octane, and n-hexadecane which are 18.40, 21.62, and 27.47 mN m−1, respectively.14 Here, because the surface energy of the obtained paper has decreased, it cannot be wetted by water, n-hexadecane, and n-octane. However, the surface energy is still higher than the liquid surface tension of n-hexane, so it can be still wetted by n-hexane.| Samples | Average contact angle (°) | |||
|---|---|---|---|---|
| Water | n-Hexane | n-Octane | n-Hexadecane | |
| GM filter paper | 0 | 0 | 0 | 0 |
| FOTS treated for 1 h | 142 | 0 | 128 | 135 |
| FOTS treated for 5 h | 145 | 0 | 128 | 138 |
| FOTS treated for 9 h | 148 | 0 | 117 | 137 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| 0.01 wt% nanocellulose/GM filter paper | 0 | 0 | 0 | 0 |
| FOTS treated for 1 h | 151 | 0 | 81 | 134 |
| FOTS treated for 5 h | 155 | 0 | 93 | 136 |
| FOTS treated for 9 h | 154 | 0 | 100 | 120 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| 0.1 wt% nanocellulose/GM filter paper | 0 | 0 | 0 | 0 |
| FOTS treated for 1 h | 134 | 0 | 53 | 90 |
| FOTS treated for 5 h | 156 | 0 | 118 | 144 |
| FOTS treated for 9 h | 154 | 0 | 106 | 140 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| 0.5 wt% nanocellulose/GM filter paper | 0 | 0 | 0 | 0 |
| FOTS treated for 1 h | 134 | 0 | 43 | 83 |
| FOTS treated for 5 h | 139 | 0 | 40 | 58 |
| FOTS treated for 9 h | 128 | 0 | 74 | 95 |
The contact angles of water, n-hexane, n-octane, and n-hexadecane on the FOTS-treated nanocellulose-modified PTFE filter papers with various loading amounts of nanocellulose and various FOTS treatment periods were also measured. As shown in Table S-1 in the ESI,† the results are similar to those of the FOTS-treated nanocellulose-modified GM filter papers. For the 0.1 wt% nanocellulose-modified PTFE filter paper, after it is treated with FOTS for 5 h, its surface also exhibits superhydrophobicity with a water contact angle as large as 153° and a water droplet on the surface becomes a marble (Fig. S-1A and B†). However, for the three non-polar liquids, its surface only repels n-hexadecane with a contact angle of 92° (Fig. S-1C†). It indicates that this surface also has superhydrophobicity but only has oleophobicity for some non-polar liquids with a larger molecular weight. For the non-polar liquids with a lower molecular weight, i.e. n-octane and n-hexane, the contact angles are 43° and 26°, respectively (Fig. S-1D and E†). Herein, it should be noted that the applied GM filter paper has an average pore diameter of 1.6 μm whereas PTFE filter paper has an average pore diameter of 0.1 μm, which could have some effect on their amphiphobic properties. In this study, the FOTS-treated nanocellulose-modified papers with the best amphiphobic properties were selected for further characterization and testing.
3.2 Surface morphology
Fig. 2A–C show the morphologies of original GM filter paper, 0.1 wt% nanocellulose-modified GM filter paper and FOTS-treated 0.1 wt% nanocellulose-modified GM filter paper, respectively. One can see that the original GM filter paper is composed of various straight fibers with a smooth surface (Fig. 2A). After modification with 0.1 wt% nanocellulose, the glass fibers are found to be significantly covered with nanocellulose (Fig. S-3A†) and the surfaces of the fibers become rough (Fig. 2B). For the FOTS-treated paper, no significant difference can be found in the morphology (Fig. 2C). However, as shown in the insets of Fig. 2, the contact angles of water and n-hexadecane on it have obvious distinctions.Fig. S-2A–C† show the morphologies of original PTFE filter paper, 0.1 wt% nanocellulose-modified PTFE filter paper and FOTS-treated 0.1 wt% nanocellulose-modified PTFE filter paper, respectively. Compared with GM filter paper, original PTFE filter paper has a denser structure with smaller PTFE fibers and lower porosity. Similar to the nanocellulose-modified GM filter paper, for the FOTS-treated nanocellulose-modified PTFE filter paper, no significant difference can be found in the morphology (Fig. S-2B and C†). However, as shown in the insets of Fig. S-2,† the contact angles of water and n-hexadecane on it have obvious distinctions, and the FOTS-treated nanocellulose-modified PTFE filter paper also shows superhydrophobicity and oleophobicity. In this study, one main advantage of loading nanocellulose on GM filter paper or PTFE filter paper is to increase the roughness of the fibers in the filter paper, which is beneficial for modifying the wettability of them from hydrophilicity and oleophilicity to superhydrophobicity and oleophobicity.1,3,11,13,15,19,24 Arbatan et al.24 fabricated superhydrophobic paper by using cellulose nanofibers as a binder to coat precipitated calcium carbonate on filter paper, followed by treatment with a solution of alkyl ketene dimer in n-heptane, and found that the water contact angle on the obtained paper was much larger than that without using cellulose nanofiber as a binder. This result is similar to the present study, in which nanocellulose is an effective material for substrate modification. It should be noted that nanocellulose can be solely used for increasing the roughness of the substrate due to its high surface area, which leads to strong adhesion on the fibers of the substrate,25 which is positive for amphiphobicity.
3.3 Chemical structure
Fig. 3 shows FT-IR spectra of (a) GM filter paper, (b) 0.1 wt% nanocellulose-modified GM filter paper, and (c) FOTS-treated 0.1 wt% nanocellulose-modified GM filter paper. Since the GM filter paper is made of 100% borosilicate glass, its main chemical composition is silicon dioxide (SiO2), boric oxide (B2O3), and other alkali oxides.26 In its FT-IR spectrum (Fig. 3-a), the detected peak at 1370 cm−1 is associated with the B–O stretching vibration band while the absorption peak at 1075 cm−1 is the asymmetric stretching vibration of Si–O–Si.27 After it is modified using nanocellulose, the absorption peaks at 3500–3200 and 2894 cm−1 are attributed to the O–H stretching and C–H stretching of nanocellulose, respectively (Fig. S-3B†).28,29 In addition, the appearance of the absorption peak at 1649 cm−1 indicates water absorption by nanocellulose. This result confirms that the modification using nanocellulose successfully increases the amount of O–H on the modified substrate. After the FOTS treatment, new absorption peaks are detected at around 1243 and 1143 cm−1 (Fig. 3B-c), which are the C–F stretching and symmetric CF2 stretching of FOTS, respectively.22 Another new absorption peak at 1072 cm−1 is associated to the Si–O–Si asymmetric stretching vibration of FOTS, indicating that FOTS has reacted with the –OH bonds of nanocellulose. Moreover, the intensity of the O–H group at 3500–3200 cm−1 is also decreased due to less –OH bonds after the amphiphobic treatment. This result confirms that the surface of the nanocellulose-modified GM filter paper has been successfully modified with FOTS, resulting in the improvement of the wettability to superhydrophobicity and oleophobicity.Fig. S-4† shows FT-IR spectra of (a) PTFE filter paper, (b) 0.1 wt% nanocellulose-modified PTFE filter paper, and (c) FOTS-treated 0.1 wt% nanocellulose-modified PTFE filter paper. As shown in Fig. S-4a,† the characteristic peaks of PTFE at 1205, 1150, and 636 cm−1 indicate stretching of the CF2 group.30 For the 0.1 wt% nanocellulose-modified PTFE filter paper, new peaks at 3500–3200, 2894, and 1649 cm−1 are detected, which indicate the existence of nanocellulose (Fig. S-4b†). However, for the FOTS-treated 0.1 wt% nanocellulose-modified PTFE filter paper, peaks corresponding to FOTS and Si–O–Si cannot be obviously detected even though the wettability has been improved from hydrophilicity and oleophilicity to amphiphobicity (Fig. S-4c†). This is because the spectral features of new structures such as Si–O–Si from the chemical vapor deposition have been hidden by the main spectral features of PTFE, i.e. at the wavelengths of 1205, and 1150 cm−1. Based on these FTIR results, the possible reaction which occurred on the nanocellulose-modified filter paper should be as shown in Fig. 4. The filter paper modified with nanocellulose can not only increase the roughness of the nanofibers, but also increase the active hydroxyl groups on the paper. With the chemical vapor deposition of FOTS a thin film composed of covalent linkages as shown in Fig. 4 leads to the amphiphobicity of the nanocellulose-modified papers.2,6,21,22,31,32
 | ||
| Fig. 4 Schematic of the chemical vapor deposition of FOTS (R = C8H13F4) on the nanocellulose-modified surface. | ||
3.4 Durability
The durability of the amphiphobic surface of the FOTS-treated 0.1 wt% nanocellulose-modified GM filter paper was evaluated by measuring the contact angle after putting it in various environments. Fig. 5A shows the contact angles of water and n-hexadecane after 6 h of soaking in various concentrations of NaCl. The results indicate that the contact angles undergo no obvious change after soaking in 1, 3, and 5 wt% NaCl and the wettability still remains at the level of superhydrophobic and oleophobic states. Fig. 5B shows the effect of environmental temperature on amphiphobicity. One can see that the amphiphobicity is almost unchanged in cold environments, and in a hot environment (50 °C) the contact angles of water and n-hexadecane decreased less than 10% but still remain in the range of amphiphobicity. Fig. 5C shows the durability of the amphiphobic surface after soaking in various solutions with different pH values. One can see that the water contact angles decrease by around 3% and 7% after 6 h of soaking in a strong acidic solution (pH = 1) and strong basic solution (pH = 14), respectively, but still remain in the range of ultrahydrophobicity while the contact angle of n-hexadecane decreased by around 3% after soaking in strong basic solution but still remained in the range of ultraoleophobicity. Fig. 5D shows the stability of the amphiphobic surface soaked in real seawater for 6, 12, and 24 h. One can see that the contact angles after 24 h of soaking decrease by only around 3% and also remain in the range of amphiphobicity.Fig. S-5† shows the durability of the amphiphobic surface of FOTS-treated 0.1 wt% nanocellulose-modified PTFE filter paper, which was also evaluated by measuring the contact angle after putting it in the same environments. Almost identical results were obtained. Thus, it can be concluded that the obtained amphiphobic filter papers have excellent durability in various environments.
Table 2 and Fig. 6 represent the contact angles of different kinds of liquids dropped on the amphiphobic surface of 0.1 wt% nanocellulose-modified GM filter paper. One can see that all contact angles of seawater, sodium chloride solution (5 wt%), distilled water (7 °C), distilled water (50 °C), sulfuric acid solution (pH = 1), sodium hydroxide solution (pH = 14), toluene, ethylene glycol, formamide, and vacuum pump oil are higher than 135°, indicating that the amphiphobicity is retained for different kinds of liquids. Similarly, as shown in Table S-2 and Fig. S-6,† for the amphiphobic surface of 0.1 wt% nanocellulose-modified PTFE filter paper, except for toluene and vacuum pump oil, the contact angles of the other liquids are larger than 120°, also indicating that amphiphobicity can be retained for different kinds of liquids.
| Liquid | Average contact angle (°) |
|---|---|
| Seawater | 153 |
| Sodium chloride solution (5 wt%) | 152 |
| Distilled water (7 °C) | 150 |
| Distilled water (50 °C) | 148 |
| Sulfuric acid solution (pH = 1) | 153 |
| Sodium hydroxide solution (pH = 14) | 151 |
| Toluene | 136 |
| Ethylene glycol | 143 |
| Formamide | 150 |
| Vacuum pump oil | 143 |
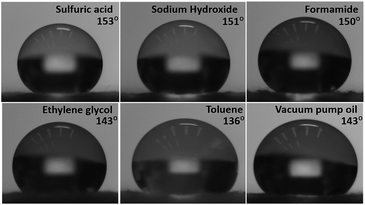 | ||
| Fig. 6 Various liquid droplets on the amphiphobic surface of FOTS treated 0.1 wt% nanocellulose-modified GM filter paper. | ||
4 Conclusions
Amphiphobic nanocellulose-modified papers with high durability have been successfully fabricated using a facile two-step method, in which nanocellulose-modified paper can be prepared through dipping filter paper such as GM filter paper and PTFE filter paper in a dilute nanocellulose dispersed solution and then, the nanocellulose-coated paper is further treated with FOTS via chemical vapor deposition. The obtained paper is found to have superhydrophobicity and oleophobicity which can repel various polar and non-polar liquids. Under the optimum conditions, the contact angles of water and n-hexadecane reach 156° and 144°, respectively on the amphiphobic surface of 0.1 wt% nanocellulose-modified GM filter paper. Furthermore, the obtained amphiphobic nanocellulose-modified papers exhibit high surface durability in several environments including at various temperatures, and in acid and alkaline solutions, salt solutions and seawater. The novelty of this work is the advantage of solely using nanocellulose, which comes from nature, to increase the roughness and the number of hydroxyl groups of filter paper for amphiphobic treatment. Meanwhile, the lower quantity of FOTS used in the reaction is another point that supports a decrease in harmfulness to the environment.Acknowledgements
This work is supported by JST and the Aomori City Government. The authors would like to thank the Daio Paper Corporation for providing nanocellulose samples. Phanthong P. gratefully acknowledges the scholarship from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.References
- H. Teisala, M. Tuominen and J. Kuusipalo, Adv. Mater. Interfaces, 2014, 1, 1300026 CrossRef.
- B. Arkles, Paint Coatings Ind. Mag., 2006 Search PubMed.
- P. Ragesh, V. A. Ganesh, S. V. Nair and A. S. Nair, J. Mater. Chem. A, 2014, 2, 14773–14797 CAS.
- B. N. Sahoo and B. Kandasubramanian, RSC Adv., 2014, 4, 22053–22093 RSC.
- J. Song and O. J. Rojas, Nord. Pulp Pap. Res. J., 2013, 28(2), 216–238 CAS.
- S. S. Latthe, A. B. Gurav, C. S. Maruti and R. S. Vhatkar, J. Surf. Eng. Mater. Adv. Technol., 2012, 2, 76–94 CAS.
- Y. Si and Z. Guo, Nanoscale, 2015, 7, 5922–5946 RSC.
- K. Liu, Y. Tian and L. Jiang, Prog. Mater. Sci., 2013, 58, 503–564 CrossRef CAS.
- G. Hayase, K. Kanamori, G. Hasegawa, A. Maeno, H. Kaji and K. Nakanishi, Angew. Chem., Int. Ed., 2013, 52, 10788–10791 CrossRef CAS PubMed.
- A. Tuteja, W. Choi, M. Ma, J. M. Mabry, S. A. Mazzella, G. C. Rutledge, G. H. Mckinley and R. E. Cohen, Science, 2007, 318, 1618–1622 CrossRef CAS PubMed.
- L. Li, V. Breedveld and D. W. Hess, ACS Appl. Mater. Interfaces, 2013, 5, 5381–5386 CAS.
- H. Mertaniemi, A. Laukkanen, J. E. Teirfolk, O. Ikkala and R. H. A. Ras, RSC Adv., 2012, 2, 2882–2886 RSC.
- A. B. D. Cassie and S. Baxter, Trans. Faraday Soc., 1944, 546–551 RSC.
- J. J. Jasper, J. Phys. Chem. Ref. Data, 1972, 1(4), 841–1009 CrossRef CAS.
- C. F. Jin, Y. F. Jiang, T. Niu and J. G. Huang, J. Mater. Chem., 2012, 22, 12562–12567 RSC.
- B. L. Peng, N. Dhar, H. L. Liu and K. C. Tam, Can. J. Chem. Eng., 2011, 89, 1191–1206 CrossRef CAS.
- Y. Habibi, L. A. Lucia and O. J. Rojas, Chem. Rev., 2010, 110, 3479–3500 CrossRef CAS PubMed.
- R. J. Moon, A. Martini, J. Nairn, J. Simonsen and J. Youngblood, Chem. Soc. Rev., 2011, 40, 3941–3994 RSC.
- G. Gonçalves, P. A. A. P. Marques, T. Trindade, C. P. Neto and A. Gandini, J. Colloid Interface Sci., 2008, 324, 42–46 CrossRef PubMed.
- J. Brassard, D. K. Sarkar and J. Perron, Appl. Sci., 2012, 2, 453–464 CrossRef CAS.
- V. A. Ganesh, S. S. Dinachali, S. Jayaraman, R. Sridhar, H. K. Raut, A. Gora, A. Baji, A. S. Nair and S. Ramakrishna, RSC Adv., 2014, 4, 55263–55270 RSC.
- N. Abidi, L. Cabrales and P. Aminayi, Moroccan J. Chem., 2015, 3(1), 167–184 Search PubMed.
- H. Jin, M. Kettunen, A. Laiho, H. Pynnonen, J. Paltakari, A. Marmur, O. Ikkala and R. H. A. Ras, Langmuir, 2011, 27(5), 1930–1934 CrossRef CAS PubMed.
- T. Arbatan, L. Zhang, X. Y. Fang and W. Shen, Chem. Eng. J., 2012, 210, 74–79 CrossRef CAS.
- D. J. Gardner, G. S. Oporto, R. Mills and M. A. S. A. Samir, J. Adhes. Sci. Technol., 2008, 22, 545–567 CrossRef CAS.
- P. Marques, US Pat., no. US 7,341,966 B2, 2008.
- M. Nolan, T. S. Perova, R. A. Moore, C. E. Beitia, J. F. M. Gilp and H. S. Gamble, J. Electrochem. Soc., 2000, 147(8), 3100–3105 CrossRef CAS.
- M. K. M. Haafiz, A. Hassan, Z. Zakaria and I. M. Inuwa, Carbohydr. Polym., 2014, 103, 119–125 CrossRef CAS PubMed.
- N. Johar, I. Ahmad and A. Dufresne, Ind. Crops Prod., 2012, 37, 93–99 CrossRef CAS.
- S. Zanini, R. Barni, R. Della Pergola and C. Riccardi, J. Phys.: Conf. Ser., 2014, 550, 012029 CrossRef.
- A. Y. Fadeev and T. J. McCarthy, Langmuir, 2000, 16, 7268–7274 CrossRef CAS.
- S. Li, H. Xie, S. Zhang and X. Wang, Chem. Commun., 2007, 4857–4859 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra24986d |
| This journal is © The Royal Society of Chemistry 2016 |