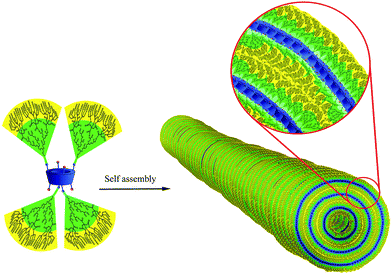
Synthesis of calixarene–polyglycerol conjugates and their self-assembly toward nano and microtubes†
Zeinab Rafieea,
Ali Kakanejadifarda,
Rahman Hosseinzadehb,
Mohammad Nematib and
Mohsen Adeli*a
aFaculty of Science, Lorestan University, Khorram Abad, Iran. E-mail: mohadeli@yahoo.com
bFaculty of Chemistry, University of Mazandaran, Babolsar, Iran
First published on 27th January 2016
Abstract
Amphiphilic hyperbranched polymers and copolymers consisting of polyglycerol, polycitric acid and a p-sulfonatocalix[4]arene core (HPCs) were synthesized and characterized. Depending on the composition of AHPs, their self-assembly in aqueous solution led to different nano and microtubes with the ability of encapsulation of anticancer drugs such as curcumin.
Calixarenes as macrocyclic oligomers with 4–20 phenol units present two defined rims and a hydrophobic core. Since they show extensive host properties for small guest molecules, calixarenes are of high interest in supramolecular science.1,2
Due to their many unique properties, calixarenes are interesting candidates for the preparation of supramolecular polymers3 and nanostructures.4 Calixarenes are able to associate different blocks of a supramolecular polymer by host–guest chemistry5 or be a part of the structure of those polymers by covalent links. In the first case, the polymer could be disassociated to its primary components reversibly and therefore it is considered as a stimuli-responsive material.6
In the second case, calixarenes are a part of the structure of the synthesized system and therefore it is possible to benefit from the properties of polymers and calixarenes as building blocks of one hybrid system.7 A polymeric structure with calixarene blocks is expected to show host–guest and all other physicochemical properties of these macrocyclic oligomers. The combination of the properties of polymers and calixarenes in one object leads to promising materials and powerful tools for future applications.8–10
p-Sulfonatocalixarenes are one of the most well-known families of water-soluble calixarene derivatives with the ability to bind to organic cations selectively, driven by the synergistic effect of additional binding sites conjugated to the rim of the cavity with an intrinsic encapsulation property.11 Inspired by the interesting host–guest and self-assembly properties of p-sulfonatocalixarenes in aqueous solutions, conjugation of high functional polymers to the rim of their cavity, opposite to the position of the sulfonate groups, results in new macromolecules with unique self-assembly behaviors and being smart functional materials.
In this work, new types of hyperbranched polymers and copolymers with a calixarene core (HPCs) have been synthesized and characterized by different methods. Depending on the time and also composition of the polymer conjugated to the calixarene, self-assembly of HPCs in aqueous solution led to nano and microtubes. Those supramolecular tubes are able to encapsulate anticancer drugs such as curcumin and therefore show a potential application for future cancer therapy.
Anionic ring opening polymerization of glycidol by p-sulfonatocalix[4]arene as an initiator led to hyperbranched polyglycerol with a calixarene unit in its focal point (calix–PG). Citric acid units were conjugated to hydroxyl functional groups of polyglycerol by a simple approach, as reported by our group previously,12 to obtain a polyglycerol–polycitric acid copolymer with a calixarene core (calix–PG–PCA) (Fig. 1).
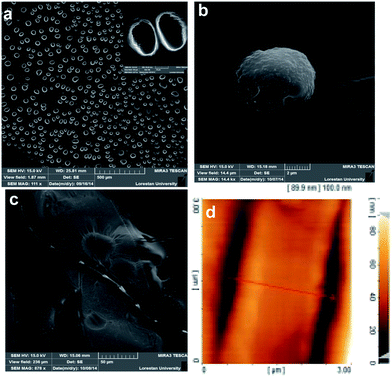
Due to the hydrophilicity of polyglycerol and the sulfonate groups and the hydrophobicity of calixarene, self-assembly of HPCs in aqueous solution resulted in a variety of nano- and micro-objects. While the sizes of the copolymers in the fresh solutions were small and they were almost in the individual forms (Fig. 4S and 5S†), their molecular self-assembly resulted in different nano- and micro-objects with different morphologies. Molecular self-assembly of calix–PG led to short nanotubes with a 30 nm diameter on average. The average thickness of the walls of the nanotubes was 10 nm and their length was varied between 70 and 300 nm (Fig. 2 and 9Sa†). A possible mechanism for the self-assembly of calix–PG is to fix calixarene in the 1,3 alternative conformation to avoid steric hindrance of polyglycerol branches and repulsive interactions between sulfonate groups and then π–π interactions between calixarene moieties. Collapse of the polyglycerol branches onto the calixarene cores, to avoid interactions with water, leads to the final assemblies.
The longitudinal association of these vesicles in turn results in the nanotubes having a cavity with a 10 nm diameter (Fig. 3). Evidence for such a mechanism comes from NMR experiments.
Since polyglycerol branches are collapsed on the calixarene rings, the proton signals for this part cannot be detected in the 1H and 13C NMR spectra (Fig. 2S†). However, after conjugation of polycitric acid to the functional groups of polyglycerol, signals of the calixarene core can be seen clearly. Repulsive interactions between negative charges of the carboxyl functional groups of the polycitric acid blocks cause an expanded conformation and therefore a slower relaxation for the calixarene moiety (Fig. 3S†). However, hydrogen bonding between citric acid units dominates the self-assembly of the calix–PG–PCA copolymer.13 By having PG–PCA branches in both sides of the calixarene, aggregation of the copolymers will be mediated to a high extent. Therefore assemblies in the form of big objects will be created. This leads to self-assemblies which are different to the previous case, calix–PG, in terms of morphologies and sizes.
Initially, it causes nucleation and formation of big spherical vesicles several micrometers in size (Fig. 4a and b). Then association of those vesicles results in big tubes with a 2 micrometer inner diameter and a several hundred micrometer length (Fig. 3, 4c and d and 6S†). Molecular self-assemblies are stable enough versus the tip of AFM to be evaluated by this method. Microtubes of around 2.5 μm in diameter are recognized by AFM (Fig. 4d). The fine structure of the wall of the microtubes shows that they consist of molecular self-assemblies situated in parallel lines with grooves of 0.4 nm in depth between them (Fig. 7S†).
Self-assembly was further investigated by looking at the assemblies using SEM and AFM at different times. As it can be seen after 6 h, assemblies are in the form of particles with a 1.5–5 μm average diameter and are 100 nm in height (Fig. 8Sa and b†). After 24 h, those particles start to assemble linearly (Fig. 8Sc–g†). The final product of such a process is to form tubes after 72 h (Fig. 9Sb–d†).
The ability of the molecular self-assemblies to encapsulate small guest molecules such as curcumin, and therefore their potential application as anticancer drug delivery systems, was also investigated (ESI,† pages 8–11). A 2/1 stoichiometric coefficient for the host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest complex between curcumin and calix–PG or calix–PG–PCA was obtained through UV-vis experiments (Fig. 10S and 11S†).
guest complex between curcumin and calix–PG or calix–PG–PCA was obtained through UV-vis experiments (Fig. 10S and 11S†).
Since encapsulation of hydrophobic guest molecules, having intrinsic fluorescence, by host systems protects the fluorophoric moiety from excited-state deactivation, which is caused by internal conversion and collisions with the water molecules from the bulk solution, this leads to an increase in the fluorescence of the guest and this effect could be used to confirm the encapsulation of the guest molecules by the host system. The rise in the fluorescence intensity of curcumin upon increasing the concentration of calix–PG and calix–PG–PCA proves the encapsulation of this drug by the tubes generated by self-assembly of the polymers and copolymers (Fig. 5).
 | ||
| Fig. 5 The fluorescence spectra of curcumin in the presence of different concentrations of molecular self-assemblies of (a) calix–PG and (b) calix–PG–PCA. | ||
The fluorescence images of the self-assemblies loaded with curcumin not only prove the encapsulation of this drug but also show longitudinal and spherical morphologies for the calix–PG and calix–PG–PCA assemblies, respectively, as is observed in different microscopy images (Fig. 12S†).
There is a direct relationship between the drug loading efficiency (LE) and size of the molecular self-assemblies. While the LE of nanotubes formed by self-assembly of calix–PG is 41%, it is 57% for the microtubes produced from calix–PG–PCA.
Release of the encapsulated curcumin from the self-assemblies was investigated at pH 5 and 7.4. It was found that the rate of release of curcumin from the self-assemblies increases by changing the pH from 7.4 to 5 dramatically (Fig. 6).
 | ||
| Fig. 6 In vitro release of encapsulated curcumin from the self-assemblies of (a) calix–PG and (b) calix–PG–PCA in PBS, at 37 °C. | ||
The higher rate of release of curcumin from the self-assemblies at pH 5 is due to their lower stability in acidic conditions. Increased hydrophilicity, due to the protonation of polymer branches at low pH and also hydrolysis of the ester bonds in the case of calix–PG–PCA, triggers dissociation of the assemblies and faster release of the encapsulated drug.
Due to the stability of those anticancer drug delivery systems at physiological pH and fast release of the drug at pH 5, they are promising systems for controlled release of therapeutic agents in the target tissues and therefore powerful tools for future cancer therapy.
In summary, new hyperbranched polymers and copolymers consisting of polyglycerol and citric acid with a calixarene core have been synthesized. The molecular self-assembly of those polymers and copolymers results in nano and microtubes with the ability of loading of curcumin as an anticancer drug. Due the pH-dependent release of this drug from the nano and microtubes, they are promising systems for future cancer therapy.
Acknowledgements
Authors would like to thank Iran National Science Foundation:INSF for the support of this work financially.Notes and references
- C. D. Gutsche and D. R. Stewart, J. Am. Chem. Soc., 1999, 121, 4136–4146 CrossRef.
- N. Basilio, V. Francisco and L. Garcia-Rio, Int. J. Mol. Sci., 2013, 14, 3140–3157 CrossRef CAS PubMed.
- V. Villari, G. Gattuso, A. Notti, A. Pappalardo and N. Micali, J. Phys. Chem. B, 2012, 116, 5537–5541 CrossRef CAS PubMed.
- Y. Liu, H. Wang, H.-Y. Zhang and P. Liang, Chem. Commun., 2004, 2266–2267 RSC.
- D.-S. Guo, K. Wang, Y.-X. Wang and Y. Liu, J. Am. Chem. Soc., 2012, 134, 10244–10250 CrossRef CAS PubMed.
- K. Wang, D.-S. Guo, X. Wang and Y. Liu, ACS Nano, 2011, 5, 2880–2894 CrossRef CAS PubMed.
- H.-h. Yu, B. Xu and T. M. Swager, J. Am. Chem. Soc., 2003, 125, 1142–1143 CrossRef CAS PubMed.
- P. Lo. Meo, G. Lazzara, L. Liotta, S. Riela and R. Noto, Polym. Chem., 2014, 5, 4499–4510 RSC.
- R. D. Dria, B. A. Goudy, K. A. Moga and P. S. Corbin, Polym. Chem., 2012, 3, 2070–2081 RSC.
- J. Ueda, M. Kamigaito and M. Sawamoto, Macromolecules, 1998, 31, 6762–6768 CrossRef CAS.
- D.-S. Guo, K. Wang and Y. Liu, J. Inclusion Phenom. Macrocyclic Chem., 2008, 62, 1–21 CrossRef CAS.
- M. Adeli, A. Kakanejadifard, M. Khani, F. Bani, R. Kabiric and M. Sadeghizad, J. Mater. Chem. B, 2014, 2, 3589 RSC.
- A. TavakoliNaeini, M. Adeli and M. Vossoughi, Nanomedicine, 2010, 6, 556–562 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra24941d |
| This journal is © The Royal Society of Chemistry 2016 |