Modifying influences of micro crystalline and nanocellulose on the gelling characteristics of poly(methacrylic acid-co-2-hydroxyethylmethacrylate)
Abstract
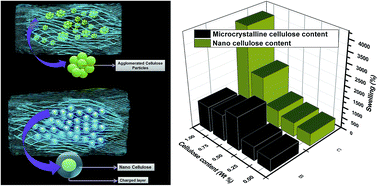
Cellulose dispersions were incorporated in the methacrylic acid (MAc)–2-hydroxyethyl methacrylate (HEMA) copolymer hydrogel matrix formed in situ and the effects of such incorporation on various properties of the resulting hydrogel were investigated by means of swelling–deswelling study, morphological study and rheological characterization. The absorption capacity of the hydrogels decreases on increasing HEMA content in the copolymer network. The introduction of cellulose, particularly nanocellulose in the MAc–HEMA co-polymer network enhances remarkably the swelling efficiency of the hydrogels. Such incorporation also affects the rheological and morphological characteristics of the hydrogels. The deposition or adsorption of cellulose in and around the pores of the polymer matrix is clearly revealed by SEM images. The cellulose based hydrogels are found to be efficient particularly with respect to the removal of cationic dyes and heavy metal ions (Cu2+ and Fe3+) from aqueous solution.

 Please wait while we load your content...
Please wait while we load your content...