An ultrafast hydrogel photocrosslinking method for direct laser bioprinting
Abstract
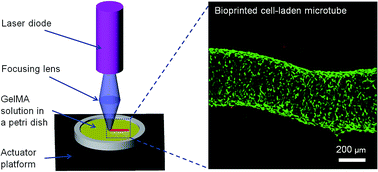
Photocrosslinking is a widely-used method to generate cell-laden hydrogels for tissue engineering. At present, it usually takes more than 30 seconds to crosslink hydrogels using UV illumination, and this delay makes it more likely that damage will occur in the DNA. With this in mind, we introduce an ultrafast photocrosslinking method using a low-cost blue laser diode. Experimental results show that a hydrogel with a diameter of 8 mm can be crosslinked using this process within 10 seconds with over 90% cell viability. Moreover, it is shown that the laser can be focused for the implementation of bioprinting. A microscale cell-laden microtube was successfully fabricated with this laser-based system, demonstrating its feasibility for bioprinting.

 Please wait while we load your content...
Please wait while we load your content...