V and Ni recovery from a vanadium-rich power plant residual ash using acid producing fungi: Aspergillus niger and Penicillium simplicissimum
Abstract
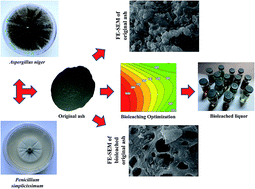
Bioleaching of V and Ni from a vanadium-rich power plant residual (PPR) ash using Aspergillus niger and Penicillium simplicissimum was investigated. To find the optimum fermentation period, prior to optimization, fungal growth was monitored through measurement of pH and produced organic acids. Preliminary tests revealed that spent-medium bioleaching, in contrast with one-step and two-step bioleaching methods, was the most efficient method, which should be optimized for enhancement of simultaneous V and Ni recovery. Pulp density, leaching temperature and leaching duration were selected as three influencing factors on metals recovery. Leaching temperature of 60 °C, leaching duration of 7 days and pulp densities of 29.2 and 32.2 g L−1 respectively for Aspergillus niger and Penicillium simplicissimum were determined as the optimal conditions. Under optimum condition V and Ni recoveries were respectively 97% and 50% using Aspergillus, while 90% of V and 49% of Ni were recovered using Penicillium. Chemical leaching tests were also conducted using commercial organic acids at equivalent values of maximum amount produced by both of the fungi. The results showed that bioleaching improved chemical leaching of V and Ni up to 15% and 12%, respectively. TCLP tests indicated that bioleaching successfully detoxified PPR ash. FE-SEM photomicrographs also confirmed high efficiency of bioleaching.

 Please wait while we load your content...
Please wait while we load your content...