Microwave-assisted synthesis of 3-sulfenylindoles by sulfonyl hydrazides using organic ionic base-Brønsted acid†
Abstract
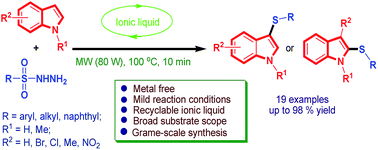
A novel, efficient, and high-yielding method was developed for the synthesis of 3-sulfenylindoles via a DBU-based ionic liquid promoted sulfenylation of indoles using sulfonyl hydrazides as a thiol surrogate. The environmentally friendly procedure, easy operation and mild reaction conditions enable the tolerance of a wide scope of functionalities as well as high reaction efficiency. The synthetic procedure is suitable for both N-protected or unprotected indoles.
- This article is part of the themed collection: Editors Collection for RSC Advances - India

 Please wait while we load your content...
Please wait while we load your content...