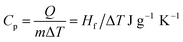Synthesis and characterization of protic ionic liquids as thermoelectrochemical materials†
T. A. Siddiquea,
S. Balamurugana,
S. M. Said*a,
N. A. Sairib and
W. M. D. W. Normazlanb
aElectrical Engineering Department, Faculty of Engineering, University of Malaya, 50603 Kuala Lumpur, Malaysia. E-mail: smsaid@um.edu.my; cheksu@gmail.com; tawsif.mme07@gmail.com
bChemistry Department, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia
First published on 2nd February 2016
Abstract
This unique work reports on the thermoelectrochemical potential of protic ionic liquid (PIL)-based electrolytes coupled with the I−/I3− redox couple. Two series of protic ionic liquids based on secondary and/or tertiary ammonium cations with the trifluoroacetate, methanesulfonate, trifluoromethanesulfonate and tosylate anions were synthesized for thermoelectrochemical cells. The complete study on PILs was carried out to determine the nature and efficiency for the generation of voltage through the electrochemical effect. The investigation was executed in a temperature range between room temperature and 90 °C. PILs show lower thermal conductivity and good ionic conductivity which leads to the success of good thermoelectric materials. The outcome was positive as our proposed PILs showed higher Se values of 420 μV K−1 obtained for TEHA TFMS than the reported values of the same I−/I3− redox couple. The most favorable thermoelectric figure of merit value (949.46 × 10−6) was achieved by BEHA TFMS. The power and the current output of the studied PILs are higher than those of some aprotic ionic liquids (AILs) reported.
Introduction
Renewable energy resources are a current global concern, given the depletion of fossil fuels, along with the detrimental effects of fossil fuel burning such as global warming and environmental pollution. Over 50% of the energy is lost as waste heat during the fossil fuel burning process for energy generation. While the dependence on fossil fuels cannot be fully overcome, thermoelectric technology provides a secondary energy generation technology which can be used in tandem with fuel engines, in order to harvest the waste heat into useful electricity.1,2Several classes of materials have been utilised as thermoelectric materials (inorganic semiconductor alloys such as bismuth telluride and lead) in terms of their performance and commercialisation development. However, such alloys have drawbacks such as high synthesis and fabrication costs, complex manufacturing processes and limited natural resources. In recent years, organic thermoelectric materials have been shown to be viable candidates for thermoelectric generation, such as PEDOT:PSS. Even more recently, ionic liquids (ILs) doped with a redox couple have been shown to demonstrate an electrochemically driven thermoelectric generation capability, which is also known as the thermoelectrochemical effect.3–7 These IL-based devices differ in operation from the semiconductor-based thermoelectrics, as they are driven by a redox electrochemical reaction which arises from a temperature gradient. Thermoelectric devices are prepared from thermoelectric cells, which are an array of thermoelectrochemical cells electrically connected in series but thermally connected in parallel. As reviewed by MacFarlane et al.,8 the thermoelectrochemical cells may directly convert thermal energy into electrical energy,9,10 which limits their operating temperature to less than 100 °C.
ILs are organic salts with melting points generally below 100 °C structured in three-dimensional networks of ions (anions and cations). These ILs may be divided into two main categories, which are protic ionic liquids (PILs) and aprotic ionic liquids (AILs).11 PILs are synthesized by proton transfer between a stoichiometric Brønsted acid to a Brønsted base.12 Some groups reported their study of thermoelectrochemical cell development using AILs3–5,7 and PILs6 as electrolytes to generate electrical energy. PILs are advantageous for the development of thermoelectrochemical devices, because it implies that only moderate solubilities are required for the most efficient devices.
The first discovered IL, ethanol ammonium nitrate (EAN), reported by Gabriel in 1888,13 was later proven to be a PIL by Walden in 1914.14 EAN was prepared by a proton transfer reaction from a Brønsted acid and a Brønsted base without using any solvents, with 12.5 °C as the melting temperature.15 The main difference between PILs and AILs is the presence of an available proton in PILs which is responsible for the extra hydrogen bonding. Thus, PILs may be comprised of neutral species which are produced by proton transfer equilibrium processes and hence do not necessarily contain fully ionic components. In 2007, MacFarlane and Seddon proposed a guideline for determining “pure ILs”, where it is necessary that the presence of neutral species should be less than 1%.16
Recently, there is an increasing interest towards PILs, as well as greater attention to AILs.11 This is due to their special properties in the presence of proton-donor and -acceptor sites as these may be used to build up a hydrogen-bonding network. In addition, the preparation of PILs is simple, as both the synthesis and purification processes are easy, less expensive, have low toxicity and are degradable.17–19 Due to their beneficial properties and potential applications, PILs are now used for fuel cells,11 organic synthesis,20 gas separation,21 biological applications,22 chromatography,15 CO2 absorption,23 self-assembly,24 as the electrolyte in batteries,24 conductors,25 as propellants or explosives,26 as catalysts in chemical reactions,27 as solvents of rare polymers28,29 and as reactants in biodiesel production.30
Knowledge of thermal and physicochemical properties is important to determine the potential applications of the synthesized PILs. Recently, researchers have been discussing these thermal properties including heat capacity, phase transition temperatures and decomposition temperatures, as well as physicochemical properties such as density, viscosity, speed of sound, refractive index and ionic conductivity in some recent publications.17,18,31–36 In these reports, most cases were investigated on a series of PILs with alkyl ammonium, hydroxyl ammonium or hydroxyalkyl ammonium-based cations and carboxylate-based anions.
In this study, two series of ammonium-based PILs have been synthesized. The aim of this study is to evaluate significant fundamental data on the thermal, physicochemical and thermoelectric properties of the BEHA and TEHA series for their potential application as thermoelectric materials. The thermal properties such as the decomposition temperature, melting temperature, heat of fusion and heat capacity of the studied PILs have been analysed. The physicochemical properties such as density, viscosity and refractive index have also been investigated. Lastly and mainly, we will discuss the thermoelectric properties, namely the ionic and thermal conductivity, the Seebeck coefficient, the figure of merit and the power and current output density of these 8 PILs in combination with the standard aqueous I−/I3− electrolyte system to evaluate the overall potentiality of PILs as thermoelectric materials.
Experimental
Materials and synthesis of PILs
Bis(2-ethylhexyl)amine (99%), tris(2-ethylhexyl)amine (99%), trifluoroacetic acid (99%), methanesulfonic acid (99%), trifluoromethanesulfonic acid (99%), and p-toluenesulfonic acid (99%) were purchased from Merck. These chemicals were used as received without any further purification. PILs are formed by proton transfer between a Brønsted acid and a Brønsted base. An equimolar amount of acid and base will be reacted. The dropwise addition of acids to the amine was carried out slowly with continuous stirring with a magnetic bar, since these reactions are highly exothermic. Hence, a slight amount of water was added to reduce the heat. The mixture was then stirred at room temperature for several hours. Strong agitation was applied in order to improve the contact between the reactants, allowing the reaction to be completed. The reaction is a simple Brønsted acid–base neutralization forming an ionic liquid. Then, water was removed by heating the mixture at 80 °C under vacuum using a rotary evaporator. The PILs were further dried at 80 °C in a vacuum oven to remove any excess water. The fully distilled and dried PIL purity was checked using 1H NMR spectroscopy.Techniques
The synthesized PILs have been confirmed using 1H NMR spectroscopy ASCEND™400 (Bruker, USA) relative to tetramethylsilane (TMS). The melting temperature and the heat of fusion of the PILs were determined using a Q20 differential scanning calorimeter (TA Instrument, USA) in the range from −100 to 70 °C. The samples were heated at a scan rate of 5 °C min−1 under a nitrogen atmosphere.Thermogravimetric analysis was performed on a TGA 4000 thermogravimetric analyzer (Perkin-Elmer, USA) under atmospheric conditions. Samples between 5 and 15 mg were heated from 20 to 650 °C under a constant heating rate of 20 °C min−1. The sample chamber had a controllable environment to allow monitoring of the degradation under a dry nitrogen atmosphere. Experimental densities of the PILs were measured by DDM 2910 (Rudolph Research Analytical, USA) in the temperature range of 20–90 °C. The apparatus is precise within 1.0 × 10−4 g cm−3, and the expanded uncertainty of the measurements was estimated to be better than 0.001 g cm−3. Calibration of the densitometer was performed at atmospheric pressure using dry air and pure water (supplied). The refractive index was measured by an RM40 refractometer (Mettler Toledo, USA). The measurement was taken at atmospheric pressure and at the temperature range between 20 and 90 °C, with a 10 °C increment. As for the viscosity study, the measurement was carried out using a Rheometer MCR301 (Anton Paar, Austria). The rheometer is a controlled stress (or controlled torque) instrument and was calibrated using standard viscosity oil. The temperature of the solution was maintained within ±0.1 °C. The viscosity was measured with an accuracy of more than 97%. All measurements for each sample were performed in triplicate, and the values were reported as an average. The measurement was taken at atmospheric pressure and at the temperature range between 20 and 90 °C, with a 10 °C increment. Ionic conductivity was measured using a DZS-708 multi-parameter analyzer from Cheetah. This was carried out at atmospheric pressure in the temperature range 25–70 °C with a 5 °C increment.
Thermal conductivity measurement was carried out using a KD2 Pro (a product of Decagon devices, Inc.) in the temperature range of 20–70 °C, with a 10 °C increment at atmospheric pressure. This experiment was verified by measuring the thermal conductivity of water. The Seebeck coefficient was obtained using the two beaker experiment method where two separate cells were connected through the salt bridge of the PIL/iodide redox couple solution. One of these two cells was heated and the other one was kept at room temperature. The Pt electrodes were used in cells and connected via a voltmeter (Agilent 34461A-6½ Digit Multimeter). The potential difference between the two cells increased with temperature at a linear rate, and Se values were obtained. The same setup of the Seebeck coefficient was used for the thermoelectrochemical device measurements. Additionally, a resistor box was connected in a parallel position with the voltmeter. This is to measure the potential at a fixed temperature difference at known resistances to calculate the current and output power of the thermoelectrochemical cells through Ohm’s law (I = V/R) and Joule’s law (P = I2R = V2R−1).
Results and discussion
Thermal stability
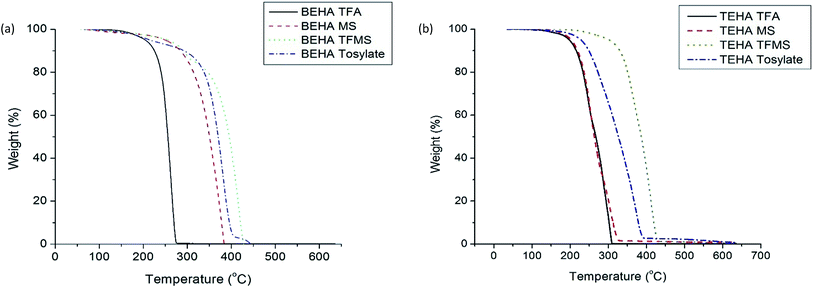
The decomposition temperature (Td) is an important property for thermoelectric materials as it reflects the thermal stability of the materials. Therefore, a high decomposition temperature is desirable for PILs as it results in a high utilization temperature range. The secondary and tertiary ammonium cations increase the decomposition temperature by increasing the branched chain of PILs. The intermolecular interaction between anions and cations is also responsible for the decomposition temperature. A very strong intermolecular interaction increases the decomposition temperature due to strong electrostatic interaction between the cation and anion. Hence more energy is required to break the chemical bonds.37The thermal stability of PILs has been evaluated using thermogravimetric analysis (TGA), and the traces of the curves are represented in Fig. 1. As a result, the decomposition temperatures are tabulated in Table 3. The decomposition temperatures are at T5% onset which means that the onset of the thermal decomposition curves is for the first 5% weight loss. Drab et al. reported that the values at T5% onset provide a more accurate decomposition temperature than the onset of the decomposition temperature.38
As a result of TGA, the decomposition temperatures of the PILs are varied from 197.8 to 302.5 °C. In both the BEHA and TEHA series, the TFA anion-doped PIL has the lowest decomposition temperature of 204.8 °C and 197.8 °C. On the other hand, the TFMS anion-doped PIL has the highest decomposition temperature at 256.2 °C and 302.5 °C of the BEHA and TEHA PIL series respectively. The thermal stability and the decomposition temperature can be correlated directly to the electrostatic force between the anion and cation.
In general, the dissociation constant of acid/anion is defined as the Ka value. The exponential numbers of the dissociation constant are converted into a normal range by taking their negative logarithm, which is defined as pKa, which represents the strength of acidity. The lower the pKa value is, the more acidic the compound is in nature. A strong acid is able to form a strong chemical bond with a cation. Hence more energy is needed to break the chemical bond. Therefore, an anion with stronger acidity exhibits a higher decomposition temperature. The pKa values of all the anions are listed in Table 2. Among these four anions, TFMS has a very low pKa value (pKa = −14) and as a result the decomposition temperature of the TFMS-containing PILs is higher than that of the other members. On the other hand, the pKa value of TFA (pKa = −0.25) is higher than that of the other anions as it is weakly acidic in nature. Hence its decomposition temperature is lower than that of the other PILs. Given that all PILs have a decomposition temperature of 200–300 °C, this will be possible for use in energy harvesting from low grade energy sources.
Melting temperature
The synthesized PILs listed in Table 1 have a broad range of melting points, from around −70.8 to 49.2 °C. In general, the melting point may be increased through increasing the packing efficiency of the ions. Several factors influence the melting points of the PILs, which has been discussed in previous literature,31,38–43 including steric hindrance, packing efficiency, hydrogen bonding, interactions between anions and cations, etc. The key factor is mainly the steric hindrance, which disrupts the packing efficiency and minimizes the hydrogen bonding of molecules.| Name | Abbreviation | |
|---|---|---|
| 1 | Bis(2-ethylhexyl)ammonium trifluoroacetate | BEHA TFA |
| 2 | Bis(2-ethylhexyl)ammonium methanesulfonate | BEHA MS |
| 3 | Bis(2-ethylhexyl)ammonium trifluoromethanesulfonate | BEHA TFMS |
| 4 | Bis(2-ethylhexyl)ammonium tosylate | BEHA tosylate |
| 5 | Tris(2-ethylhexyl)ammonium trifluoroacetate | TEHA TFA |
| 6 | Tris(2-ethylhexyl)ammonium methanesulfonate | TEHA MS |
| 7 | Tris(2-ethylhexyl)ammonium trifluoromethanesulfonate | TEHA TFMS |
| 8 | Tris(2-ethylhexyl)ammonium tosylate | TEHA tosylate |
In the PILs of the BEHA series, among the 4 anions doped, the MS anion-doped PILs show a very low melting point (−11.2 °C) whereas all the remaining PILs show higher melting temperatures. BEHA MS contains the methyl sulphonyl group which gives more flexibility for the PILs. The remaining anions TFA and TFMS contain highly electronegative fluorine atoms which have the capability to form hydrogen bonds with the cationic hydrogen (from the secondary amine) atom more strongly. Hence, BEHA TFA and BEHA TFMS possess higher melting temperatures of 49.2 °C and 10.8 °C respectively. The aromatic group containing BEHA tosylate is highly rigid compared to the other members of this series hence it has a higher melting temperature (24.9 °C) than BEHA MS and BEHA TFMS.
However, in the case of the TEHA series, TEHA TFMS has a lower melting point (−69.9 °C) than TEHA MS (−62.8 °C) due to the interaction between anions and cations. Fluorine is more electronegative, thus it contributes a greater number of hydrogen bonds. When comparing TEHA TFA (−70.8 °C) and TEHA TFMS (−69.9 °C), the presence of the methyl sulfonyl group gives a higher electronegative effect, which is more favourable for hydrogen bonding, resulting in a higher melting temperature. However, the aryl ring in TEHA tosylate provides a higher molecular weight (483 g mol−1), which causes a higher melting temperature (−58.8 °C).
In a comparison study of the BEHA and TEHA series, the anions of BEHA contribute to a very high melting temperature compared to that of the TEHA series. This may be due to the steric hindrance of the alkyl group, which is higher for the tertiary ammonium (TEHA) than the secondary ammonium (BEHA). Hence, this affects the molecular close packing of the PILs of the TEHA series. Melting temperature is an inherent property of materials, and a low melting temperature is a required criterion for PILs. Therefore, a low melting temperature is desirable, but it does not have a direct effect on the thermoelectric performance of the PILs. From the DSC thermogram, the BEHA and TEHA series do not show a glass transition temperature (Tg) in between our experimental temperatures of −100 °C to 100 °C.
Heat of fusion
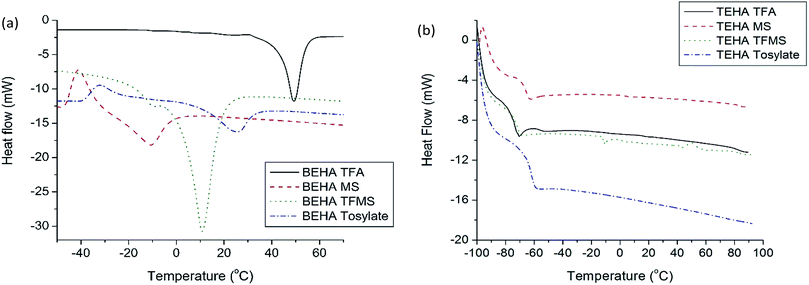
Heat of fusion (Hf) is a specific amount of heat which is required to convert one gram of a solid to a liquid state at its melting point without changing the temperature. This energy basically breaks down the solid bonds, meanwhile leaving a significant amount of energy for the association of the intermolecular forces in the liquid state. Hence, a lower Hf is desirable for thermoelectrics as it requires lower energy.Heat of fusion is calculated from the peak area at the melting temperature from the DSC curve. Thus, the sharper the peak is, the greater the heat of fusion of the analysed compound.
| Hf = Q/m |
The values of heat of fusion of the synthesized PILs are given in Table 3 which are obtained from the DSC curve (Fig. 2). Heat of fusion depends on various factors such as functional groups, alkyl chain length, hydrogen bonds (within cations and interaction with anions), steric hindrance effects, electronegativity effects of the anions, cation volumes, molecular weight, and interaction forces between anions and cations.44,45
Clear endothermic peaks are observed in the DSC thermogram for the BEHA series which provides the information of the heat of fusion. Among the four PILs of the BEHA series, the TFA anion-doped PIL has the highest heat of fusion (70.4 J g−1). The next candidate in this series is the TFMS anion-doped PIL which possesses a heat of fusion of 32.75 J g−1. Both the electronegative fluorine-substituted PILs (BEHA TFA and BEHA TFMS) have higher values, as fluorine increases the binding energy of the PILs. Hence, higher energy is required to overcome the binding energy. BEHA MS possesses a heat of fusion of 23.66 J g−1.44,45 The lowest value, 14.37 J g−1, was obtained for the tosylate-doped PILs (BEHA tosylate). On the other hand, when a similar cation such as TEHA is used, it shows different results from the BEHA group due to the anion–cation interactions between the TEHA cation and the TFA, MS, TFMS and tosylate anions.45
In the presence of similar anions like TFA, MS, TFMS and tosylate, the BEHA group has a higher heat of fusion value compared to that of the TEHA group due to the steric hindrance effect. For example, BEHA TFA has a Hf value of 70.4 J g−1 which is higher than that of TEHA TFA (3.01 J g−1). The secondary amine of BEHA results in less hydrogen bonding, which leads to higher energy.45
Heat capacity
Heat capacity is the required heat to increase the temperature of 1 g substance by 1 K. In other words, it can also be defined as the stored energy per molecule before the temperature increases. The heat capacity is calculated by the ratio of heat of fusion and the temperature difference of the melting peak. The unit is J g−1 K−1. The calculated values of Cp from the DSC curve are given in Table 3.The heat capacity values of the BEHA series are in the range of 0.827 to 11 J g−1 K−1 and the TEHA series are in the range of 0.094 to 0.422 J g−1 K−1, and the mechanism of the thermoelectrochemical cells is to produce energy from the difference of temperature between the hot side and the cold side as it creates a temperature gradient. Therefore, the increment of temperature is deeply related to the mechanism of thermoelectricity as increasing the temperature of the hot side is required to increase the temperature gradient in thermoelectrochemical cells. Generally, materials with a smaller heat capacity will require less energy to increase the temperature of the material. From Table 2, it is clear that BEHA MS and BEHA tosylate possess the lowest heat capacity at 0.827 J g−1 K−1 and 0.952 J g−1 K−1 respectively. Minimal energy is required to increase the temperature by 1 K. The member of the series with the next lowest heat capacity is BEHA TFMS (3.68 J g−1 K−1) as it needs little excess energy to boost up the temperature every 1 K for thermoelectrochemical cells. BEHA TFA has a very high heat capacity (11 J g−1 K−1) among the formulations presented. The anions containing fluorine atoms have a higher heat capacity than the other members and the presence of a greater alkyl chain length in cations decreases the heat capacity, as TEHA has a lower heat capacity than the BEHA series.
| PILs | Mw (g mol−1) | Td (°C) | Tm (°C) | Hf (J g−1) | Cp (J g−1 K−1) |
|---|---|---|---|---|---|
| a Mw, Td, Tm, Hf and Cp are the molecular weight, decomposition temperature, melting temperature, heat of fusion and heat capacity respectively. | |||||
| BEHA TFA | 327 | 204.8 | 49.2 | 70.4 | 11 |
| BEHA MS | 309 | 250.0 | −11.2 | 23.66 | 0.827 |
| BEHA TFMS | 363 | 256.2 | 10.8 | 32.75 | 3.68 |
| BEHA tosylate | 385 | 215.4 | 24.9 | 14.37 | 0.952 |
| TEHA TFA | 425 | 197.8 | −70.8 | 3.01 | 0.418 |
| TEHA MS | 407 | 202.4 | −62.8 | 6.11 | 0.422 |
| TEHA TFMS | 461 | 302.5 | −69.9 | 3.51 | 0.293 |
| TEHA tosylate | 483 | 228.3 | −58.8 | 0.89 | 0.094 |
Thermal conductivity
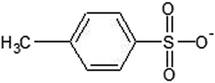
The thermal conductivity results of the PILs at 20 to 70 °C are tabulated in Table 4 and plotted in Fig. 3 as a function of temperature in the range of 20 to 70 °C, where they follow the normal trend of increasing with the increase of temperature.| Temperature (°C) | BEHA TFA | BEHA MS | BEHA TFMS | BEHA tosylate | TEHA TFA | TEHA MS | TEHA TFMS | TEHA tosylate |
|---|---|---|---|---|---|---|---|---|
| Thermal conductivity, κ (W m−1 K−1) | ||||||||
| 20 | 0.0365 | 0.039 | 0.035 | 0.033 | 0.0375 | 0.039 | 0.036 | 0.041 |
| 70 | 0.0395 | 0.0415 | 0.04 | 0.03667 | 0.04 | 0.042 | 0.0395 | 0.046 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Density, ρ (g cm−3) | ||||||||
| 20 | — | 0.9806 | 1.0635 | 0.9995 | 0.9553 | 0.9387 | 1.0164 | 0.9648 |
| 90 | 0.9351 | 0.9330 | 1.0101 | 0.9523 | 0.9000 | 0.8914 | 0.9676 | 0.9193 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Viscosity, η (Pa s) | ||||||||
| 20 | — | 1.07 | 0.622 | 1.74 | 0.357 | 2.41 | 1.72 | 4.3584 |
| 90 | 0.0109 | 0.032 | 0.0241 | 0.0435 | 0.0124 | 0.0498 | 0.0483 | 0.0944 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Ionic conductivity, σ (mS cm−1) | ||||||||
| 25 | 1.444 | 1.22 | 2.45 | 0.753 | 2.27 | 0.837 | 1.382 | 0.667 |
| 70 | 2.34 | 2.26 | 4.93 | 1.627 | 3.85 | 1.635 | 2.9 | 1.388 |
 | ||
| Fig. 3 Thermal conductivity of the synthesized PILs in combination with 0.05 M I−/I3− at various temperatures: (a) BEHA series and (b) TEHA series. | ||
It is also noted that the effect of temperature is very negligible in some of the PILs.
The figure of merit ZT decreases with high thermal conductivity at any ΔT. Therefore, a lower thermal conductivity is desired for thermoelectrochemical cells where it increases ΔT and could be maintained across the device. The greater alkyl chain length in the cations increases the thermal conductivity, as the thermal conductivity depends strongly on the IL cation alkyl chain length. This is due to the dimensions of the IL bulk nanostructure being controlled by the cation alkyl chain consisting of the charged (ordered domains) and the uncharged (disordered domains) regions. As the dimensions of the disordered domains are controlled by the cation alkyl chain, it limits the thermal conductivity.46 From all the values of the thermal conductivity at various temperatures of the PILs, it is also noticeable that the thermal conductivity is not strongly dependent on the IL anion.46 Additionally, another point is noticed which is that the presence of the fluorine and sulfonyl functional group in the anions decreases the thermal conductivity. Despite this, the presence of benzene also increases the thermal conductivity. If the above points are considered, then it can be said that the fluorination of anions and a lower alkyl chain length are desirable criteria for lower thermal conductivity as well as for higher ionic conductivity.
In the presence of the I−/I3− redox couple, the studied PILs demonstrate thermal conductivity in the range between 0.033 and 0.041 W m−1 K−1 at 20 °C which is significantly lower than water (0.67 W m−1 K−1), MPN (0.12 W m−1 K−1), [C2mim][NTF2] (0.12 W m−1 K−1), and [C2mim][B(CN)4] (0.19 W m−1 K−1).5 From this discussion, it could be easily said that the PILs are very good candidates for thermoelectric materials with respect to their thermal conductivity.
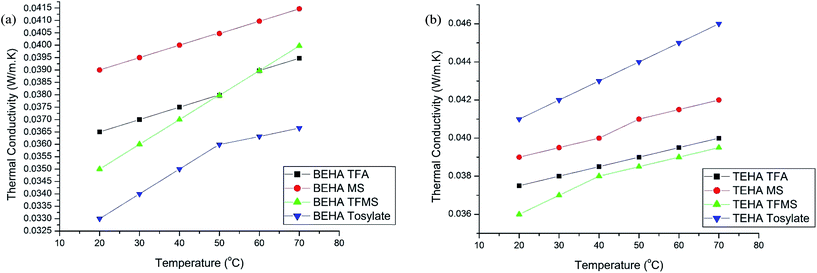
Density
The density values of the synthesized PILs at 20 to 90 °C are tabulated in Table 4. The findings indicate that the BEHA and TEHA series demonstrate temperature-dependent behaviour, where the density decreases linearly with temperature increment as shown in Fig. 4. The density of the synthesized PILs is affected by the packing of the ions, the size and shape of the ions and interactions between the ions.47 The density decreases as the alkyl chain length increases in the cations,48,49 which is the reason behind the higher density of BEHA than TEHA. In both series, the TFMS anion-containing PILs have a higher density value which may be due to the high molecular weight of the anions and two functional groups (fluorine and sulfonate) in the structure.47 A decreasing trend of density has been observed while decreasing the molecular weight of the anions, except for TFMS.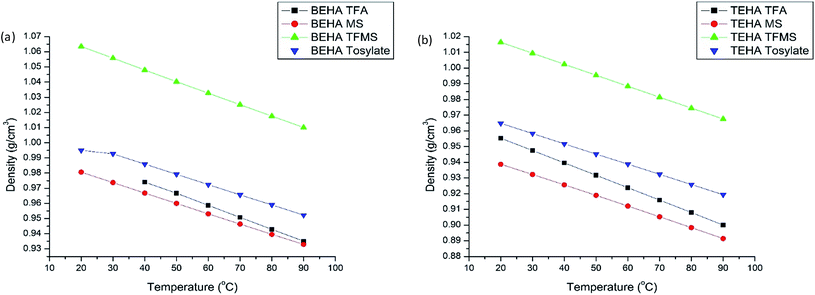 | ||
| Fig. 4 Densities of the synthesized PILs at different temperatures: (a) BEHA series and (b) TEHA series. | ||
Viscosity
Since the operation of a thermoelectrochemical cell is done by mass transfer of the solution/ions from the hot to the cold side, the viscosity of the solution is critical. Ionic conductivity is directly affected by viscosity; the ionic conductivity decreases with the increase of viscosity.7 Viscosity depends on the ion–ion interactions which mainly involve hydrogen bonding and van der Waals interactions.48 The viscosity of ILs increases with the increment of alkyl chain length as this will provide stronger van der Waals interactions.50 It is desirable to have low viscosities and high ionic conductivities for thermoelectric applications. The viscosities of the synthesized PILs at 20 to 90 °C are tabulated in Table 4.Fig. 5 shows the viscosity of the BEHA and TEHA series. For the BEHA series, the decreasing order of viscosity follows the trend BEHA tosylate > BEHA MS > BEHA TFMS > BEHA TFA, decreasing with the increase of temperature. A step decrease was observed while increasing the temperature from 20 °C to 50 °C. With a further increase of temperature, the viscosity of the PILs was not affected. In this series, the starting temperature of BEHA TFA was observed from 50 °C because of its solid nature. Interestingly, BEHA TFA has low viscosity which is not affected by temperature. It maintains a stable viscosity regardless of temperature. In the TEHA series, the decreasing order of viscosity of the PILs is as follows: TEHA tosylate > TEHA MS > TEHA TFMS > TEHA TFA. In both series, the same trend was observed with decreasing viscosity with respect to the anion dopant in the PILs. Furthermore, all members constantly decrease the viscosity with respect to temperature until 50 °C (except for TEHA TFA). On further increase of the temperature, a stable viscosity is maintained until 90 °C.
 | ||
| Fig. 5 Viscosities of the synthesized PILs at different temperatures: (a) BEHA series and (b) TEHA series. | ||
As noted from the BEHA series, the TFA-doped PIL possesses a unique property, where its viscosity is not affected by the temperature from 20 °C to 90 °C. The fluorine-substituted anionic dopant-containing PILs show very low viscosity among the rest of the members. Amongst the fluorine-substituted anions, the ionic size also plays a role, where the low molecular weight TFA has low viscosity compared to the higher molecular weight of TFMS. This concept is in good agreement with the Ohno et al. report.41
Ionic conductivity
Higher ionic conductivity is desirable for thermoelectric applications as it increases the figure of merit ZT. The ionic conductivity at 25 to 70 °C is tabulated in Table 4 and plotted in Fig. 6 in the range of 25 to 70 °C, where it is confirmed that the ionic conductivity increases with temperature as the normal trend.7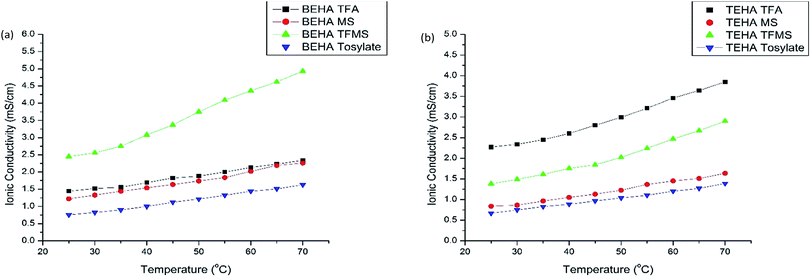 | ||
| Fig. 6 Ionic conductivity of the synthesized PILs in combination with 0.05 M I−/I3− at various temperatures: (a) BEHA series and (b) TEHA series. | ||
Amongst all the PILs, the PILs containing fluorine atoms in the anions have a higher ionic conductivity as fluorine is more electronegative than hydrogen. The sulfonyl functional group in the anions also helps the PILs to have a higher ionic conductivity than the acetate functional group does. The presence of a benzene ring in the anions decreases the ionic conductivity remarkably which is not a positive sign. On the other hand, a large alkyl chain length in the cations decreases the ionic conductivity. Therefore, it can be concluded that fluorination of the anions and a lower alkyl chain length in the cations are highly desirable in PILs for thermoelectrochemical applications.
The PILs of our study with a 0.05 M I−/I3− redox couple have an ionic conductivity in the range of 0.66 to 2.45 mS cm−1 at 25 °C and 1.38 to 4.95 mS cm−1 at 70 °C, which is higher than that of the AILs [HMIM][I], [PMIM][I], and [BMPY][BF4] reported by Uhl et al.7 where the ionic conductivities are 0.2, 1.6, and 2.4 mS cm−1 at 25 °C respectively. Therefore, PILs could be good candidates for thermoelectrochemical cells.
Seebeck coefficient
The Seebeck coefficient and the maximum voltage of the PILs of this study with the combination of the 0.05 M I−/I3− redox couple solution are tabulated in Table 5 and the Seebeck coefficients were calculated from Fig. 7. The maximum voltages (Vmax) for the PILs are in the range of 7.0 to 15.2 mV. The Se values are 1.5 to 4 times greater when the solvents are PILs (182 to 420 μV K−1) instead of DMSO (119 μV K−1), which is a positive indication for the PILs as thermoelectric materials.| PIL | Seebeck coefficient (μV K−1) | Vmax (mV) | ZTmax ×10−6 |
|---|---|---|---|
| BEHA TFA | 340 | 11.68 | 758.04 |
| BEHA MS | 410 | 14.27 | 432.86 |
| BEHA TFMS | 370 | 14.89 | 949.46 |
| BEHA tosylate | 376 | 15.18 | 253.65 |
| TEHA TFA | 182 | 7.03 | 281.23 |
| TEHA MS | 337 | 13.5 | 281.66 |
| TEHA TFMS | 420 | 14.92 | 548.68 |
| TEHA tosylate | 352 | 14.55 | 192.79 |
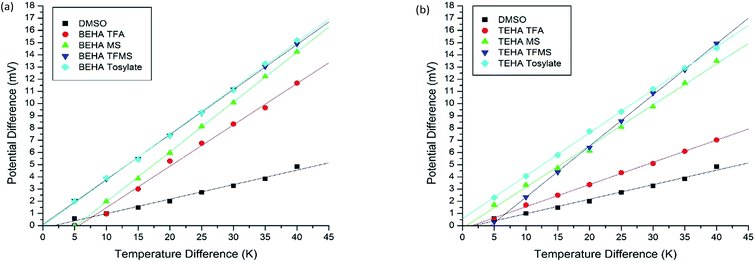 | ||
| Fig. 7 Seebeck coefficient measurements of the synthesized PILs in combination with 0.05 M I−/I3−: (a) BEHA series and (b) TEHA series. | ||
This Seebeck coefficient is directly related to the reaction entropy of the redox couple which is responsible for creating the potential difference across the thermoelectrochemical cells in the presence of the temperature gradient. The relation is as follows:
| ∂E(T)/∂T = Se = ΔSorc/nF |
In comparison with the commonly used solid-state material Bi2Te3, which has a Se of −287 μV K−1, all the PILs in this study have a higher Se (between 337 to 420 μV K−1) except for TEHA TFA (182 μV K−1). All eight PILs with a 0.05 M I−/I3− solution also have higher Se values than the AILs [C2mim][BF4] (260 μV K−1), [P4,4,4,6][NTf2] (170 μV K−1), [C2mim][NTf2] (154 μV K−1), [C4mpyr][NTf2] (60 μV K−1), [C2mim][B(CN)4] (94 μV K−1), and [P2,2,2,(101)][NTf2] (30 μV K−1) with 0.4 M I−/I3− solution, as reported by Abraham et al., even though these solutions contained a higher concentration of the I−/I3− redox couple. The Se of the AILs increased with the concentration of I−/I3− as in the reported work.3 The Se values of the studied PILs are also higher than that of [EMIM][CF3SO3] (364 μV K−1), [HMIM][I] (−130 μV K−1), and [PMIM][I] (−190 μV K−1).7 TEHA TFMS has the maximum Seebeck coefficient (420 μV K−1). It can be expected from the above discussion that PILs could be a good alternative formulation to thermoelectric materials.
Figure of merit
The efficiency of a thermoelectric material depends on the figure of merit ZT.| ZT = σSe2T/κ |
The ZT values in the temperature range of 30 to 65 °C of all the PILs are plotted in Fig. 8 and the maximum ZT values are tabulated in Table 5.
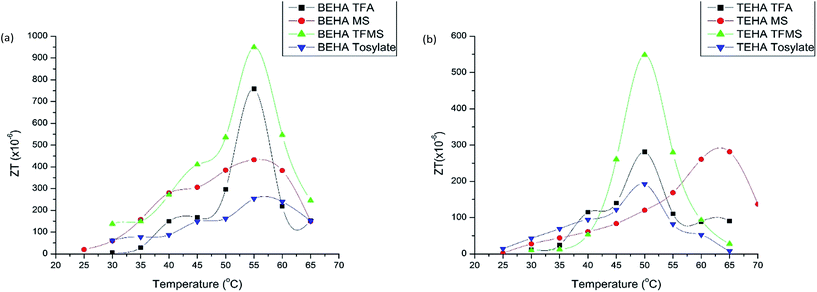 | ||
| Fig. 8 ZT of the synthesized PILs in combination with 0.05 M I−/I3− at various temperatures: (a) BEHA series and (b) TEHA series. | ||
From the above equation of ZT, it is clear that ZT is directly proportional to the ionic conductivity and Seebeck coefficient, as well as inversely proportional to thermal conductivity. Hence, the desirable properties are high ionic conductivity, a high Seebeck coefficient and low thermal conductivity. PILs containing TFMS anions give the highest ZT values in both the BEHA (949.46 × 10−6) and TEHA (548.68 × 10−6) cases, as they have lower thermal conductivity, higher ionic conductivity and a higher Seebeck coefficient. On the other hand, the tosylate PILs give the lowest ZT values (BEHA 253.65 × 10−6 and TEHA 192.79 × 10−6) as they have higher thermal conductivity, lower ionic conductivity and a lower Seebeck coefficient. The BEHA series normally gives higher ZT values than the TEHA series as the thermal conductivity is higher, and the ionic conductivity and Seebeck coefficient are lower in the TEHA group than in the BEHA group.
The maximum ZT values of our studied PILs are between 190 × 10−6 to 950 × 10−6, which are 1.5 to 46 times higher than some AILs like [C2mim][BF4] (130 × 10−6), [C2mim][NTf2] (39 × 10−6), [C4mpyr][NTf2] (33 × 10−6), [C2mim][B(CN)4] (28 × 10−6), and [C2mim][DCA] (21 × 10−6).4 From these ZT values, the efficiency of PILs as thermoelectric materials can be easily understood and they may be used for future thermoelectrochemical applications such as electrolytes.
Power and current output density
The power and current output density were calculated from the measured potential values (V) and the known resistance (R) using Ohm’s law (I = V/R) and Joule’s law (P = I2R = V2R−1). The diameter of the Pt electrode is 1 mm. The maximum values of power and current output density are tabulated in Table 6.| PIL | Thot (°C)/Tcold (°C) | Pmax (μW m−2) | Imax (mA m−2) |
|---|---|---|---|
| BEHA TFA | 50/25 | 2.13 | 123 |
| BEHA MS | 50/25 | 23.3 | 75 |
| BEHA TFMS | 50/25 | 43.2 | 129 |
| BEHA tosylate | 50/25 | 18.7 | 234 |
| TEHA TFA | 50/25 | 8.55 | 228 |
| TEHA MS | 50/25 | 15.2 | 531 |
| TEHA TFMS | 50/25 | 21.2 | 270 |
| TEHA tosylate | 50/25 | 6.66 | 294 |
The calculated values are plotted in Fig. 9 and 10 for the power and current output density respectively and the trends of power and current output density with potential follow the same as that in previous literature.4–7 Abraham et al. reported that 0.05 M Fe(CN)63−/Fe(CN)64− in [chaoline][DHP] PIL results in a 0.1 mW kg−1 power density when Pt was used as an electrode and it is independent of the solute–solvent ratio when the solvent is water.6 The aim of this study is to determine the eight different newly synthesized PIL potentialities in thermoelectrochemical cells. Hence the experiments were carried out at a fixed solvent–solute ratio.
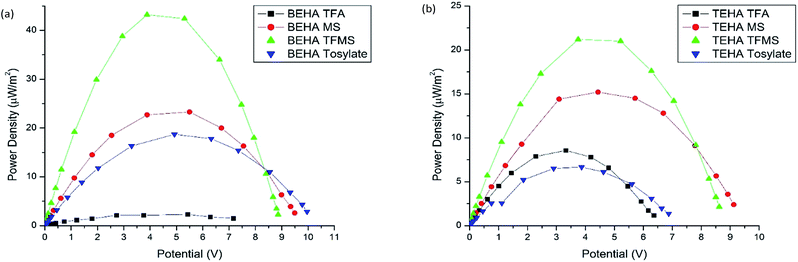 | ||
| Fig. 9 Thermoelectrochemical cell power output density versus potential plots of the synthesized PILs in combination with 0.05 M I−/I3− at 25/50 °C (Tcold/Thot): (a) BEHA series and (b) TEHA series. | ||
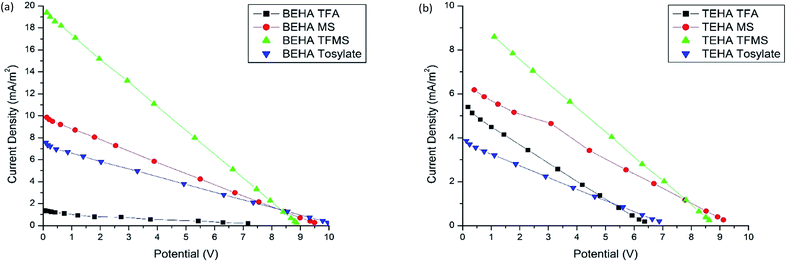 | ||
| Fig. 10 Thermoelectrochemical cell current output density versus potential plots of the PILs in combination with 0.05 M I−/I3− at 25/50 °C (Tcold/Thot): (a) BEHA series and (b) TEHA series. | ||
From Ohm’s and Joule’s laws, it is noticeable that the current (I) is directly proportional to the potential (V), and power (P) is directly proportional to the current (I) as well as to potential (V). Again, this current is directly related to the ionic conductivity as higher conductivity results in a higher current flow and lower conductivity results in a lower current flow.
The maximum power density values of the studied PILs are from 2.13 to 43.2 μW m−2. Between the BEHA and TEHA cations, BEHA has higher power output densities when the anions are the same, as the potential (V) (Table 5) is higher in the case of the BEHA group except for BEHA TFA (2.13 μW m−2) and TEHA TFA (8.55 μW m−2). On the other hand, when the cations are the same, TFMS has a higher power output than MS due to the higher output potential of TFMS, and TFA has a lower power output than TFMS, as TFA results in the lowest potential. Hence, among all the PILs, BEHA TFMS has the highest power density of 43.2 μW m−2 as it results in an almost higher potential of 14.89 mV, and BEHA TFA has the lowest power density of 2.13 μW m−2 as it results in an almost lower potential of 11.68 mV.
The maximum current output density values of the studied PILs are in the range of 75 to 531 mA m−2. When the cations are similar as in BEHA, TFMS has a greater current density than TFA due to the higher ionic conductivity of BEHA TFMS (3.75 mS cm−1) than that of BEHA TFA (1.879 mS cm−1). BEHA TFMS also has a higher ionic conductivity than BEHA MS (1.738 mS cm−1), which results in the same order of current output density. On the other hand, TEHA MS has a higher current density than that of TEHA TFMS, which is not supported by their ionic conductivity as the ionic conductivity of TEHA TFMS (2.02 mS cm−1) is higher than that of TEHA MS (1.226 mS cm−1). TEHA MS has the greatest current density of 531 mA m−2 and BEHA MS has the lowest current density of 75 mA m−2.
The power and the current output of the studied PILs are higher than some of the AILs such as [BMIM][BF4], [EMIM][CF3SO3], [HMIM][I], [EAN][NO3], [PMIM][I] and [BMPY][BF4] which results in the power output range of 0.02 to 0.7 μW and current output of 4 to 80 μA .7 Hence, the PILs’ performance as an electrolyte in thermoelectrochemical devices is also noticeable and could not be ignored.
Conclusions
The understanding of thermal and physical properties of the synthesized ammonium-based PILs is vital for their design and evaluation as thermoelectrochemical devices. Ammonium-based PILs have high potentiality for energy applications due to their basicity which leads to high ionic conductivity. A secondary and tertiary ammonium-based series (BEHA and TEHA) of the PILs have been studied. The physical properties and stability with respect to the temperature of the PILs performed suitably for thermoelectric materials. The BEHA series has higher Se values than the TEHA series. The maximum obtained Se value is 420 μV K−1, which is the highest reported in PILs in a I−/I3− redox couple. As a result, the power and the current output of the studied PILs are higher, which shows that the PIL electrolyte coupled with the I−/I3− redox couple is an attractive formulation for energy harvesting thermoelectrochemical generators.Acknowledgements
The authors are grateful to the University of Malaya Centre of Ionic Liquids (UMCIL) for assistance in the PIL synthesis and characterizations. This work has been financially supported by HIR (UM.C/625/1/HIR/MOHE/ENG/29), UMRG (RP006F-13SES), UMRG (RP023B-13AET), Science Fund (SF020-2013) and FRGS (FP011-A).References
- H. Kleinke, Chem. Mater., 2009, 22, 604–611 CrossRef.
- J. Doherty, Fossil Fuels: Examination and Prediction of Future Trends, 2012 Search PubMed.
- T. J. Abraham, D. R. MacFarlane and J. M. Pringle, Chem. Commun., 2011, 47, 6260–6262 RSC.
- T. J. Abraham, D. R. MacFarlane, R. H. Baughman, L. Jin, N. Li and J. M. Pringle, Electrochim. Acta, 2013, 113, 87–93 CrossRef CAS.
- T. J. Abraham, D. R. MacFarlane and J. M. Pringle, Energy Environ. Sci., 2013, 6, 2639–2645 CAS.
- T. J. Abraham, D. R. MacFarlane, R. H. Baughman, N. Li, Y. Chen and J. M. Pringle, MRS Proceedings, 2013, 1575, mrss13-1575-vv04-08 CrossRef.
- S. Uhl, E. Laux, T. Journot, L. Jeandupeux, J. Charmet and H. Keppner, J. Electron. Mater., 2014, 43, 3758–3764 CrossRef CAS.
- D. R. MacFarlane, N. Tachikawa, M. Forsyth, J. M. Pringle, P. C. Howlett, G. D. Elliott, J. H. Davis, M. Watanabe, P. Simon and C. A. Angell, Energy Environ. Sci., 2014, 7, 232–250 CAS.
- T. Quickenden and Y. Mua, J. Electrochem. Soc., 1995, 142, 3985–3994 CrossRef CAS.
- R. Hu, B. A. Cola, N. Haram, J. N. Barisci, S. Lee, S. Stoughton, G. Wallace, C. Too, M. Thomas and A. Gestos, Nano Lett., 2010, 10, 838–846 CrossRef CAS PubMed.
- N. M. Talavera-Prieto, A. G. Ferreira, P. N. Simões, P. J. Carvalho, S. Mattedi and J. A. Coutinho, J. Chem. Thermodyn., 2014, 68, 221–234 CrossRef CAS.
- T. L. Greaves, A. Weerawardena, I. Krodkiewska and C. J. Drummond, J. Phys. Chem. B, 2008, 112, 896–905 CrossRef CAS PubMed.
- M. Hirao, H. Sugimoto and H. Ohno, J. Electrochem. Soc., 2000, 147, 4168–4172 CrossRef CAS.
- P. Walden, Bull. Acad. Imp. Sci. St.-Petersbourg, 1914, 1800 Search PubMed.
- C. F. Poole, J. Chromatogr. A, 2004, 1037, 49–82 CrossRef CAS PubMed.
- D. R. MacFarlane and K. R. Seddon, Aust. J. Chem., 2007, 60, 3–5 CrossRef CAS.
- K. Kurnia, C. Wilfred and T. Murugesan, J. Chem. Thermodyn., 2009, 41, 517–521 CrossRef CAS.
- M. Iglesias, R. Gonzalez-Olmos, I. Cota and F. Medina, Chem. Eng. J., 2010, 162, 802–808 CrossRef CAS.
- J. Sierra, E. Martí, A. Mengíbar, R. González-Olmos, M. Iglesias, R. Cruañas and M. Garau, Effect of new ammonium based ionic liquids on soil microbial activity, in, 5th Society of Environmental Toxiology and Chemistry Word Congress, August 2008, pp. 3–7 Search PubMed.
- K. K. Laali and V. J. Gettwert, J. Org. Chem., 2001, 66, 35–40 CrossRef CAS PubMed.
- L. A. Blanchard, Z. Gu and J. F. Brennecke, J. Phys. Chem. B, 2001, 105, 2437–2444 CrossRef CAS.
- K. Fujita, D. R. MacFarlane and M. Forsyth, Chem. Commun., 2005, 4804–4806 RSC.
- X. Yuan, S. Zhang, J. Liu and X. Lu, Fluid Phase Equilib., 2007, 257, 195–200 CrossRef CAS.
- W. Tamura-Lis, L. Lis and P. Quinn, J. Phys. Chem., 1987, 91, 4625–4627 CrossRef CAS.
- M. A. Susan, A. Noda, S. Mitsushima and M. Watanabe, Chem. Commun., 2003, 938–939 RSC.
- M. Picquet, I. Tkatchenko, I. Tommasi, P. Wasserscheid and J. Zimmermann, Adv. Synth. Catal., 2003, 345, 959–962 CrossRef CAS.
- T. Jiang, H. Gao, B. Han, G. Zhao, Y. Chang, W. Wu, L. Gao and G. Yang, Tetrahedron Lett., 2004, 45, 2699–2701 CrossRef CAS.
- N. Bicak, J. Mol. Liq., 2005, 116, 15–18 CrossRef CAS.
- H.-M. Choi and I. Kwon, Ind. Eng. Chem. Res., 2010, 50, 2452–2454 CrossRef.
- M. J. Earle, N. V. Plechkova and K. R. Seddon, Pure Appl. Chem., 2009, 81, 2045–2057 CrossRef CAS.
- U. Domanska and R. Bogel-Lukasik, J. Phys. Chem. B, 2005, 109, 12124–12132 CrossRef CAS PubMed.
- J.-P. Belieres and C. A. Angell, J. Phys. Chem. B, 2007, 111, 4926–4937 CrossRef CAS PubMed.
- M. Anouti, M. Caillon-Caravanier, C. Le Floch and D. Lemordant, J. Phys. Chem. B, 2008, 112, 9406–9411 CrossRef CAS PubMed.
- V. C. H. Álvarez, N. Dosil, R. Gonzalez-Cabaleiro, S. Mattedi, M. Martin-Pastor, M. Iglesias and J. M. Navaza, J. Chem. Eng. Data, 2010, 55, 625–632 CrossRef.
- A. Pinkert, K. L. Ang, K. N. Marsh and S. Pang, Phys. Chem. Chem. Phys., 2011, 13, 5136–5143 RSC.
- M. Mahrova, M. Vilas, A. N. Domínguez, E. Gómez, N. Calvar and E. Tojo, J. Chem. Eng. Data, 2012, 57, 241–248 CrossRef CAS.
- T. Erdmenger, J. Vitz, F. Wiesbrock and U. S. Schubert, J. Mater. Chem., 2008, 18, 5267–5273 RSC.
- D. M. Drab, M. Smiglak, J. L. Shamshina, S. P. Kelley, S. Schneider, T. W. Hawkins and R. D. Rogers, New J. Chem., 2011, 35, 1701–1717 RSC.
- M. Yoshizawa, W. Xu and C. A. Angell, J. Am. Chem. Soc., 2003, 125, 15411–15419 CrossRef CAS PubMed.
- G.-H. Tao, L. He, N. Sun and Y. Kou, Chem. Commun., 2005, 3562–3564 RSC.
- H. Ohno and M. Yoshizawa, Solid State Ionics, 2002, 154, 303–309 CrossRef.
- H. Ohno, M. Yoshizawa and W. Ogihara, Electrochim. Acta, 2004, 50, 255–261 CrossRef CAS.
- A. Bagno, C. Butts, C. Chiappe, F. D’Amico, J. C. Lord, D. Pieraccini and F. Rastrelli, Org. Biomol. Chem., 2005, 3, 1624–1630 CAS.
- Z. Zhang, A. A. Salih, M. Li and B. Yang, Energy Fuels, 2014, 28, 2802–2810 CrossRef CAS.
- E. A. Turner, C. C. Pye and R. D. Singer, J. Phys. Chem. A, 2003, 107, 2277–2288 CrossRef CAS.
- T. Murphy, L. M. Varela, G. B. Webber, G. G. Warr and R. Atkin, J. Phys. Chem. B, 2014, 118, 12017–12024 CrossRef CAS PubMed.
- T. L. Greaves and C. J. Drummond, Chem. Rev., 2008, 108, 206–237 CrossRef CAS PubMed.
- Z. B. Zhou, H. Matsumoto and K. Tatsumi, Chem.–Eur. J., 2005, 11, 752–766 CrossRef CAS PubMed.
- Z.-B. Zhou, H. Matsumoto and K. Tatsumi, Chem. Lett., 2004, 33, 1636–1637 CrossRef CAS.
- P. Bonhote, A. P. Dias, N. Papageorgiou, K. Kalyanasundaram and M. Grätzel, Inorg. Chem., 1996, 35, 1168–1178 CrossRef CAS PubMed.
- J. Agar and W. Breck, Trans. Faraday Soc., 1957, 53, 167–178 RSC.
- T. Licht and N. Swendeman, J. Electrochem. Soc., 1959, 106, 616–625 CrossRef.
- E. L. Yee, R. J. Cave, K. L. Guyer, P. D. Tyma and M. J. Weaver, J. Am. Chem. Soc., 1979, 101, 1131–1137 CrossRef CAS.
- T. Quickenden and C. Vernon, Sol. Energy, 1986, 36, 63–72 CrossRef CAS.
- T. Ikeshoji, Bull. Chem. Soc. Jpn., 1987, 60, 1505–1514 CrossRef CAS.
- R. Hu, B. Cola, N. Haram, J. Barisci, S. Lee, S. Stoughton, G. Wallace, C. Too, M. Thomas and A. Gestos, Nano Lett., 2010, 10, 838–846 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR graphs and tabulated data of physical and electrochemical properties. See DOI: 10.1039/c5ra24835c |
| This journal is © The Royal Society of Chemistry 2016 |