Variations of lignin–lignin and lignin–carbohydrate linkages from young Neosinocalamus affinis bamboo culms
Bing Zhang,
Gen-Que Fu,
Ya-Shuai Niu,
Feng Peng*,
Chun-Li Yao* and
Run-Cang Sun
Beijing Key Laboratory of Lignocellulosic Chemistry, Beijing Forestry University, Beijing 100083, China. E-mail: fengpeng@bjfu.edu.cn; chunliyao2006@163.com
First published on 29th January 2016
Abstract
Three lignin–carbohydrate complex (LCC) preparations were isolated to elucidate the variations of chemical linkages during growth in the early development stages of Neosinocalamus affinis bamboo culms. A combination of chemical composition analysis and FT-IR characterization indicated that the Neosinocalamus affinis bamboo contained lignin of typical G–S–H type, and glucuronoarabinoxylan hemicelluloses. The NMR technique was used for investigating the substructures of the LCC preparations, as well as quantifying the relative amounts of chemical linkages. The results revealed that changes of β-5′ and phenyl glycoside linkages were identical to the value of S/G ratio, and increased with the maturation of the bamboo. Whereas the amounts of β-O-4′ and β-1′ linkages presented a reductive tendency when the S/G ratio was increased. The relative amounts of β–β′, benzyl ether and benzyl ester linkages were in line with the content of carbohydrates, namely, they were increased firstly and then decreased with the development of the young bamboo culms. All of these findings will provide a theoretical basis for elucidation of the mechanism of plant cell growth, as well as the utilization of young Neosinocalamus affinis bamboo culms.
Introduction
Carbohydrates and lignin make up major portions of biomass cell walls, in which carbohydrates mainly consist of two substances, cellulose and hemicelluloses.1–3 Cellulose is a long linear homogenous polysaccharide polymerized by β-1,4-glucopyranoside.4,5 On the contrary, hemicelluloses are kinds of heterogeneous polysaccharides, containing diverse monosaccharides such as arabinose, galactose, glucose, xylose, mannose, glucuronic acid, and galacturonic acid, depending on the plant species.6 Due to the existence of aromatic rings, the structure of lignin is quite different from that of carbohydrates. Lignin is an irregular aromatic polymer composed by phenylpropane units of the guaiacyl (G), syringyl (S), and p-hydroxyphenyl (H) types.7 As constructive composition and nutrition supplying supplements, cellulose, hemicellulose, and lignin play important roles in the normal growth and development of plants.In addition to the self-crosslinking, there are numerous evidences that lignin and hemicelluloses, perhaps as well as cellulose are covalently linked, forming a special compound currently called lignin–carbohydrate complex (LCC).4,8–11 Many publications have reported that linkages between lignin and carbohydrates also exist in chemical pulp.12,13 The occurrence of the linkages creates significant problems in effective isolation of lignin fraction from lignocelluloses, and it is one of the most important reasons preventing selective separation of the components in biorefining process.14 For example, bleaching is often used to remove the lignin during chemical pulping process, making the cellulosic pulp more suitable for the subsequent use. Thus, understanding the interaction between lignin and carbohydrates is of great interest for economic and efficient removal of the lignin in bleaching process. The arrangement of the main components and their interaction in the cell wall are also useful in the functionalization of biomass materials and their applications.4
Researches in these years have shown that there are mainly three types of LCC linkages in plants, which are considered to be phenyl glycoside, benzyl ether, and benzyl ester.4,9,11 In order to study the LCC linkages, all methods for analysis require firstly isolation of LCC preparations from biomass materials. Early studies summarized that the LCC preparations could be classified as carbohydrate-rich LCC, including Björkman LCC and similar ones, as well as enzymatic LCC fractions, and lignin-rich LCC, such as crude milled wood lignin and milled wood enzymatic lignin.14 Over the past few decades, investigators have devoted themselves to study both carbohydrate-rich LCC and lignin-rich LCC of softwood and hardwood. Attentions on LCC preparations of gramineous plants have seldom regarded, especially bamboo materials. Infrequent findings demonstrate that LCC linkages in non-wood plants are structurally different from that in wood plants due to the incorporation of hydroxycinnamates into the cell wall. According to the previous references,15–17 ferulic acid is esterified linked to hemicelluloses and etherified linked to lignin, forming a type of lignin–ferulate–hemicelluloses complex. In addition, the peculiar non-wood substance p-coumaric acid is also esterified linked to hemicelluloses or lignin. The existence of ferulic and p-coumaric acids make the lignin and carbohydrates in gramineous plants present a more complicated crosslinking structure.
Lignin structure and its interaction are also important to understanding LCC preparations. It is reported that the linkages between lignin and carbohydrates are vitally important related to the structure of lignin, especially the proportion of G–S–H units in the LCC preparations.18 The major linkages among lignin are aryl ether and C–C linkages, such as β-O-4′, β–β′, β-5′, and β-1′.19 Earlier lignin structural characterization of Arundo donax discovered that the structure and lignin type were varied among maturity stages and morphological regions (internodes, nodes, root, and foliage).20 Generally, as a predominant linkage, the amount of aryl ether largely depends on the configuration of lignin, which is characterized as S/G ratio. Similarly, the amounts of LCC linkages were usually determined by the S/G ratio to a large degree.18
Commonly, LCC preparation is only a part of the whole intact LCC. The structure of LCC preparation is various when using different isolation methods.21–25 A targeted approach to extract the LCC preparation can make it possible for us to better understand the specific constitute of the plants cell-wall. Furthermore, the structural characterization of LCC preparation is important in the elucidation of the mechanism of plant cell growth.26 In the present study, LCC preparations enriched in lignin–carbohydrate were isolated from 2-month-, 4-month-, and 6-month-old Neosinocalamus affinis bamboo for extrapolating the variation of bamboo chemical linkages during its early stages of development. These works will provide a theoretical basis for utilization of Neosinocalamus affinis bamboo. Subsequently, the chemical and structural changes of the bamboo LCC preparations were characterized by high performance anion exchange chromatography (HPAEC), Fourier transform infrared (FT-IR) spectroscopy, and two dimensional heteronuclear single-quantum coherence (2D-HSQC) spectroscopy.
Materials and methods
Materials
The bamboo (Neosinocalamus affinis) materials for this study were collected once every two months (2, 4, and 6 month after being unearthed, respectively) in 2014 from Sichuan Province, China. The fresh culms were dried in sunlight immediately after removing leaves and shells. The dried specimens were ground into small pieces, and the 40–60 mesh particles were sifted for further experiment. The screened powder was extracted with ethanol/toluene (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) in a Soxhlet apparatus for 6 h to remove the wax. Whereafter, the dewaxed sample was subjected to milling in a planetary ball mill using ZrO2 balls for 8 h. The appearance of the three ball-milled samples is shown in Fig. 1, and it was quite various due to different growth stages.
2, v/v) in a Soxhlet apparatus for 6 h to remove the wax. Whereafter, the dewaxed sample was subjected to milling in a planetary ball mill using ZrO2 balls for 8 h. The appearance of the three ball-milled samples is shown in Fig. 1, and it was quite various due to different growth stages.
 | ||
| Fig. 1 Different appearance of the 2-month- (a), 4-month- (b), and 6-month-old (c) ball-milled bamboo powder. | ||
Isolation of LCC preparations and hemicelluloses
The LCC preparations were isolated from the 2-month-, 4-month-, and 6-month-old ball-milled bamboo powder (labeled as L2, L4, and L6, respectively) according to the method of Balakshin et al.27 The scheme is illustrated in Fig. 2 and the operations are as follows: the ball-milled bamboo powder was extracted by dioxane (96%, v/v) with a solid to liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (g mL−1) at room temperature for 24 h under stirring. The procedure was repeated three times and all the combined filtrate and washing liquid were concentrated at reduced pressure to obtain the crude MWL. The crude MWL was then dissolved in acetic acid (90%, v/v) with a solid to liquid ratio of 1
20 (g mL−1) at room temperature for 24 h under stirring. The procedure was repeated three times and all the combined filtrate and washing liquid were concentrated at reduced pressure to obtain the crude MWL. The crude MWL was then dissolved in acetic acid (90%, v/v) with a solid to liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (g mL−1). The filtrate was precipitated in the water drop by drop. The supernatant was collected by centrifugation and then evaporated under vacuum. 30 mL water was added to remove trace amount of acetic acid and the step was repeated at least three times.
20 (g mL−1). The filtrate was precipitated in the water drop by drop. The supernatant was collected by centrifugation and then evaporated under vacuum. 30 mL water was added to remove trace amount of acetic acid and the step was repeated at least three times.
 | ||
| Fig. 2 Scheme for extraction of LCC preparations (L2, L4, and L6) from 2-month-, 4-month-, and 6-month-old ball-milled bamboo powder. | ||
As all the lignin is widely recognized mainly being covalently linked to hemicelluloses, the corresponding bamboo hemicelluloses were subsequently extracted for better understanding the composition of the carbohydrates moiety. Dimethyl sulfoxide was selected as the solvent for the isolation of hemicelluloses without cleaving chemical linkages, the method was adapted from the literature.28 The delignified sample (holocellulose) was dissolved in dimethyl sulfoxide with a solid to liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 (g mL−1) at 80 °C for 7 h under stirring. After treatment of dimethyl sulfoxide, the filtrate was concentrated at reduced pressure, and then mixed with three volumes of 95% ethanol. The precipitated hemicelluloses were obtained by centrifugation and freeze-dried. The hemicelluloses obtained from the 2-month-, 4-month-, and 6-month-old bamboo culms were marked as H2, H4, and H6, respectively.
25 (g mL−1) at 80 °C for 7 h under stirring. After treatment of dimethyl sulfoxide, the filtrate was concentrated at reduced pressure, and then mixed with three volumes of 95% ethanol. The precipitated hemicelluloses were obtained by centrifugation and freeze-dried. The hemicelluloses obtained from the 2-month-, 4-month-, and 6-month-old bamboo culms were marked as H2, H4, and H6, respectively.
Analytical methods
The main chemical components (carbohydrates, acid-insoluble lignin, and acid-soluble lignin) of the three LCC preparations were determined according to the method published by Sluiter et al.29 The sugars in the hemicelluloses extracted were determined by HPAEC according to the procedures described in a previous literature.30 The physicochemical properties and structural features of the LCC preparations were evaluated and characterized by FT-IR and 2D-HSQC spectroscopy as previously reported.31,32Results and discussion
Chemical composition analysis
The main chemical composition of the LCC preparations were carbohydrates, acid-insoluble lignin, and acid-soluble lignin. The relative contents of the main chemical composition are given in Table 1. In aggregate, the three components were obtained with a yield of 3.7, 3.9, and 2.1% in relative to the oven dry weight of the ball-milled bamboo powder. As shown in Table 1, the changes of the acid-insoluble and acid-soluble lignin exhibited a paradoxical intention with age increasing. The development of the young bamboo culms from 2-month-old to 6-month-old resulted in a significant increase of acid-insoluble lignin, while a remarkable decrease of acid-soluble lignin, which was explained by the structural change of the lignin. As a consequence, the variations of the carbohydrates in L2, L4, and L6 were negatively consistent with the total lignin. In addition, the content of acid-soluble lignin was inconceivable higher than those of the acid-insoluble lignin in L2, L4, and L6, suggesting that the immature bamboo culms contained a relatively higher acid-soluble lignin content than mature bamboo culms.| Preparationsa | Yieldb | Main chemical composition (% of relative content) | Sugar (% of relative molar content) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Acid-insoluble lignin | Acid-soluble lignin | Carbohydrates | Arabinose | Galactose | Glucose | Xylose | Mannose | ||
| a L2, L4, and L6 represent the 2-month-, 4-month-, and 6-month-old LCC preparation, respectively.b Relative to the oven dry weight of the ball-milled bamboo powder (%). | |||||||||
| L2 | 3.7 | 7.9 | 55.9 | 36.2 | 3.7 | 0.8 | 86.4 | 3.6 | 5.5 |
| L4 | 3.9 | 10.5 | 38.2 | 51.3 | 3.0 | 0.9 | 77.1 | 15.1 | 3.9 |
| L6 | 2.1 | 19.9 | 32.1 | 48.0 | 3.8 | 1.0 | 59.2 | 32.9 | 3.1 |
The relative molar contents of sugar in three LCC preparations are also listed in Table 1. As can be seen from Table 1, glucose (86.4–59.2%) was the predominant sugar composition among the five kind of sugars, suggesting the presence of high proportion of glucans. Xylose appeared as the second major sugars, comprising of 3.6–32.9% of the total sugars, which was the largest component of hemicelluloses. Arabinose (3.0–3.8%), galactose (0.8–1.0%), and mannose (3.1–5.5%) were identified as small but indispensable amount.
Dimethyl sulfoxide was firstly used as a suitable solvent for investigating the original structure of hemicelluloses from plant materials by Hägglund et al.33 Three fractions of hemicelluloses were hydrolyzed to determine their sugar constituents, and the results are listed in Table 2. It is reported that the hemicelluloses from Neosinocalamus affinis mainly consisted of glucuronoarabinoxylans.34 As can be seen from Table 2, xylose was the predominant sugar composition (59.1–94.3%) in H2, H4, and H6, suggesting the presence of high proportion of xylans. The linear β-1,4-D-Xylp backbone is substituted by α-Araf and α-D-glucopyranosyl uronic units, and the ratios of arabinose to xylose (Ara/Xyl) and glucuronic acid to xylose (GlcA/Xyl) represent for the degree of linearity or branching of hemicelluloses.6,28 According to the results of Ara/Xyl and GlcA/Xyl ratios from Table 2, the degree of linearity in hemicelluloses were increased with bamboo ages.
| Sugarb (% of relative molar content) | Ara/Xylc | GlcA/Xyld | |||||
|---|---|---|---|---|---|---|---|
| Ara | Gal | Glu | Xyl | GlcA | |||
| a H2, H4, and H6 represent the 2-month-, 4-month-, and 6-month-old hemicelluloses, respectively.b Ara, Gal, Glu, Xyl, and GlcA represent the arabinose, galactose, glucose, xylose, and glucuronic acid respectively.c Represents molar ratio of arabinose to xylose.d Represents molar ratio of glucuronic acid to xylose. | |||||||
| H2a | 9.3 | 5.6 | 25.6 | 59.1 | 0.4 | 0.16 | 0.007 |
| H4a | 4.7 | 1.2 | 4.8 | 88.7 | 0.6 | 0.05 | 0.007 |
| H6a | 3.7 | 0.7 | 1.1 | 94.3 | 0.2 | 0.04 | 0.002 |
FT-IR analysis
The FT-IR spectra of the three LCC preparations obtained from Neosinocalamus affinis bamboo are shown in Fig. 3. In the functional group region (>1800 cm−1), all of the three spectra show a strong absorption at 3321 cm−1, which is attributed to O–H stretching vibrations in the aromatic and aliphatic hydroxyl groups. Other identical absorptions at 2929 and 2862 cm−1 are produced by C–H stretching vibrations from methyl and methylene groups.35 Apparently, the similar spectra of L2, L4, and L6 in Fig. 3 (region 1800 to 700 cm−1) show bands characteristic of gramineous lignin (G–S–H type) as follows: 1656 and 1721 cm−1 (conjugated and nonconjugated carbonyl groups),36 1594, 1510, and 1421 cm−1 (aromatic ring),37 1123 and 824 cm−1 (S), as well as 1022 and 882 cm−1 (G).38,39 Bands at 1510 and 1452 cm−1 are useful in demonstrating the presence of lignin in lignocelluloses, since they do not overlap with bands from carbohydrates. However, the peak at 1452 cm−1 can be observed in spectra L2 and L4 seems not appeared in the L6, suggesting that the structure of the lignin part in the three preparations have some difference. To some extent, the strength of the absorption at 1721 cm−1 represents the linkages between lignin and hemicelluloses, which seems to be more intense in L4 and L6 than in L2.38,39 The obvious absorption observed at 1236 cm−1 is assigned to C–O stretching of condensed guaiacyl.40 In addition, the small signal at 1170 cm−1 is assigned to the C–O stretching in C–O–C glycosidic linkages, and the contribution of C–OH bending from arabinoxylans.41 Signals ranging from 1100–1000 cm−1 and other regions are typical of glucans and xylans, which are overlapped by the lignin signals. The 882 cm−1 band also indicated the β-type of the polysaccharides linkages.42 In general, the difference between the FT-IR spectra of L2, L4, and L6 can be explained by the various composition of lignin and carbohydrates, which are shown in Table 1.Quantification of LCC preparations with 2D-HSQC spectra
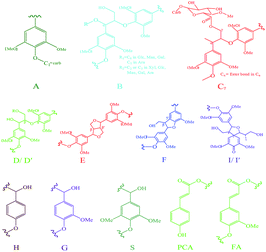
NMR technique is widely used to provide important information of the complex molecule structure from lignocellulosic materials, particularly 13C–1H response of lignin monomer and monosaccharide, as well as their associated linkages.27,31,43–45 In this study, the LCC preparations isolated from 2-month-, 4-month-, and 6-month-old bamboo were characterized by a 400 MHz NMR spectrometer, and the 13C–1H 2D-HSQC spectra are depicted in Fig. 4 (L2, L3, and L4, respectively). The marks in Fig. 4 are explained as follows: A: phenyl glycoside, B: benzyl ether, Cα: α-ester, Cγ: γ-ester, D (D′): β-O-4′ substructure, E: β–β′ resinol substructure, F: β-5′ phenylcoumaran substructure, I (I′): β-1′ spirodienone substructure, G: guaiacyl unit, H: p-hydroxyphenyl unit, PCA: p-coumarate unit, FA: ferulate unit, Glc: β-D-glucopyranoside unit, X: β-D-xylopyranoside unit, Rα: α-reducing end carbohydrate units, and Rβ: β-reducing end carbohydrate units. The corresponding LCC linkages and lignin substructures are illustrated in Fig. 5. Quantification of the major lignin–carbohydrate and lignin–lignin linkages is a very important index to characterize the structural difference among the three LCC preparations. In the present study, the quantification method was adopted according to the literature.31 The amounts of the main LCC and lignin linkages were calculated by the mean of three parallel samples and the results were expressed as how much linkages per 100 aromatic rings. The formula are as follows:| IC9 units = 0.5IS2,6 + IG2 + 0.5IH2,6 (grass lignin) |
| AX = IX/I9 × 100 |
Major LCC linkages
It is reported that phenyl glycoside, benzyl ether, and benzyl ester linkages are the main types of LCC linkages in plants.4,27 The three types of linkages are identified by 2D-HSQC NMR from the bamboo LCC preparations L2, L3, and L4.A noticeable signal of carbohydrates CH1 involved in phenyl glycoside linkages is given at 100.3/4.84 ppm (Fig. 4, area A in L2, L4, and L6) according to model compound data.46 It is noteworthy that there is only one signal in area A (δC/δH 104–99/5.2–4.8) assigned to phenyl glycoside linkages, indicating that various carbohydrate units form phenyl glycoside linkages are with low content and below the detection level. Combined with the sugar analysis of LCC preparations and hemicelluloses, the visible signal is probably originated from lignin–xylan or lignin–glucan type phenyl glycoside linkages. In addition, it is well known that cellulose is composed of β-glucose linked through β-1,4-glycosidic bonds. The prominently high proportion of glucose in L2, L4, and L6 (seen in Table 1) indicated that some cellulose may link to lignin.47
The integration of the signal in area A (Fig. 4) gives an approximate value for the amount of phenyl glycoside linkages in the LCC preparations of 7.1–11.3 per 100 aromatic ring (Ar) (Table 3). As shown in Table 3, the LCC preparation L6 contained the highest relative amount of phenyl glycoside linkages among the three samples with the lowest yield of 2.1% (Table 1). It was about 1.4 and 1.6 times higher than those in L4 and L6, separately, which present an increased tendency with bamboo growth. This was because that the content of xylose in hemicelluloses was dramatically increased with the maturation of the bamboo (Table 2), and the bamboo LCC preparation probably contained mainly lignin–xylan type phenyl glycoside linkages.
Benzyl ether linkages are kinds of relatively stable LCC bonds which even not hydrolyzed in alkali environment.27 The 2D-HSQC spectrum regularly shows signals in the area of 81.0–80.0/5.1–4.5 ppm assigned to CHα in benzyl ether LCC bonds based on data from corresponding model compounds.48 Similarly to the signals for phenyl glycoside LCC linkages, the intensity of the signals can be integrated to estimate the amount of benzyl ether linkages (δC/δH 81.0–79.0/5.05–4.55 of area B circled by dotted line in Fig. 4). However, the area B shows few signals. In order to calculate the integration, area B was enlarged, and the picture is depicted in the upper-left corner of L2, L3, and L4 in Fig. 4. The relative amounts of benzyl ether linkages are listed in Table 3. In contrast to phenyl glycoside linkages, it was a little smaller, and was increased in the sequence L6 < L2 < L4 from 1.7/100 Ar in L6 to 2.5/100 Ar in L4.
Benzyl ester linkages between benzyl units in lignin and glucuronic acid moieties in carbohydrates are considered to be the main type of ester LCC linkages.24,49 As shown in Fig. 4, the cross-peak originated from α-ester linkages at 75.0/6.10 ppm in area Cα (circled by dotted line) is zoomed and located in the left column just blow picture B. The cross-peak originated from γ-ester linkages at 65–62/4.5–4.0 ppm is also revealed in Fig. 4, and it is delineated as area Cγ. However, it is not accurate by using the amount of γ-ester linkages for the representative of benzyl ester linkages, considering the various types of lignin γ-esters, such as ferulate and coumarate types. For this reason, the signal in area Cα was integrated to estimate the amount of benzyl ester linkages. The relative amount of benzyl ester linkages is list in Table 3. Although the sugar analysis of the three LCC preparations revealed that almost no glucuronic acid existing, the 2D-HSQC spectra shows CH2 and CH3 signals of glucuronic acid at 72.23/3.39 and 73.52/3.41 ppm, respectively (Fig. 4). In addition, glucuronic acid existed in the branch of hemicelluloses according to the result of sugar analysis from Table 2 was probably linked to lignin by benzyl ester linkages. Similarly to the benzyl ether linkages, the relative amount of benzyl ester linkages shown in Table 3 was increased in the row L6 < L2 < L4 from 0.6/100 Ar in L6 to 2.1/100 Ar in L4. The LCC preparation L6 contained only 0.6/100 Ar benzyl ester linkages, which was due to the low amount of GlcA/Xyl ratio (0.002) identified by the sugar analysis of H6 (Table 2).
Major lignin linkages
Except for main LCC linkages, the lignin linkages well help us acquire more information about the structure of the bamboo LCC preparation. Various lignin linkages, such as β-O-4′, β–β′, β-5′, and β-1′ could be assigned by the 2D-HSQC spectra according to the abundant databases in a number of previous references.50–54 The relative amounts of the major lignin substructures among the bamboo LCC preparation were investigated and quantitated based on the 2D-HSQC spectra, and the results are listed in Table 3.As expected, the β-O-4′ aryl ether linkages were the predominant interunit linkages in lignin, with an amount of 46.3, 46.2, and 24.6/100 Ar in L2, L3, and L4, separately. Comparing with β-O-4′ linkages, the amount of β–β′, β-5′, and β-1′ were actually much lower, ranging from 0.2 to 0.7/100 Ar. As shown in Table 3, it was noteworthy that the amounts of the four linkages in L6 appeared quite different from the other two samples. The β-O-4′, β–β′, and β-1′ linkages in L6 were much lower than that in L2 and L4, while the β-5′ linkages was much higher than that in L2 and L4, which was due to the different structure of lignin in L6. Additionally, the S/G ratio of L2, L4, and L6 was calculated to be 0.16, 0.20, and 0.49, respectively (Table 3). The value of S/G was increased with the age of the bamboo, suggesting that the mature bamboo always contained much higher amount of S type lignin than the immature bamboo. As a previous publication mentioned, higher S/G ratio always results in a high content of β-O-4′ linkages in lignin macromolecule.55 However, the inconsistency in this study could be explained by the existence of high proportion of acid-soluble lignin.
In addition of the major LCC linkages and lignin linkages, various signals from the associated carbohydrates could also be found in the 2D-HSQC spectra (Fig. 4), including xylopyranoside units (X), glucopyranoside units (Glc), glucuronic acid units (U), and other carbohydrates signals which was overlapped and interlaced.56
Conclusions
The LCC preparations L2, L4, and L6 were extracted from 2-month-, 4-month-, and 6-month-old Neosinocalamus affinis bamboo culms for elucidating the variations of chemical linkages during the growth in their early development stages. The major linkages between lignin–lignin and lignin–carbohydrate largely depend on the proportion of the chemical composition. The percentage of the acid-insoluble lignin in the LCC preparation was increased with maturation of the bamboo, while the percentage of the acid-soluble lignin was decreased with the increasing age of the bamboo. Young Neosinocalamus affinis bamboo LCC preparations contained lignin of typical G–S–H type, and the content of acid-soluble lignin was higher than the acid-insoluble lignin. The S/G ratio varied in consistent with the proportion of the acid-soluble lignin. In other words, the mature bamboo culms contained higher content of S type lignin than the immature bamboo culms. The changes of β-5′ and phenyl glycoside linkages were identical with the value of S/G ratio, whereas the amounts of β-O-4′ and β-1′ presented a reductive tendency when the S/G ratio was increased. The relative amounts of β–β′, benzyl ether, and benzyl ester linkages were in line with the content of carbohydrates, namely, they were increased firstly and then decreased with the development of the young bamboo culms.Acknowledgements
This work was supported by Fundamental Research Funds for the Central Universities (JC2015-03), National Natural Science Foundation of China (31470417), Ministries of Education (NCET-13-0670), Author of National Excellent Doctoral Dissertations of China (201458), and the National Program for Support of Top-notch Young Professionals.Notes and references
- A. Ebringerová, Z. Hromádková and T. Heinze, Adv. Polym. Sci., 2005, 186, 1–67 CrossRef.
- P. Peng and S. Diao, Carbohydr. Polym., 2014, 112, 701–720 CrossRef CAS PubMed.
- T. E. Timell, Wood Sci. Technol., 1982, 16, 83–122 CrossRef CAS.
- D. Fengel and G. Wegener, in Wood: chemistry, ultrastructure, reactions, Walter de Gruyter, 1983 Search PubMed.
- M. Lewin and I. S. Goldstein, in Wood structure and composition, ed. M. Dekker, 1991 Search PubMed.
- F. Peng, P. Peng, F. Xu and R. C. Sun, Biotechnol. Adv., 2012, 30, 879–903 CrossRef CAS PubMed.
- M. Y. Balakshin, E. A. Capanema and H. M. Chang, in Characterization of lignocellulosic materials, 2009, pp. 148–170 Search PubMed.
- K. V. Sarkanen and C. H. Ludwig, in Lignins: occurrence, formation, structure and reactions, 1971 Search PubMed.
- O. Grushnikov and V. Elkin, in Advances and problems of lignin chemistry [in Russian], Nauka, Moscow, 1973 Search PubMed.
- W. G. Glasser, R. A. Northey and T. P. Schultz, in Lignin: historical, biological, and materials perspectives, American Chemical Society, Washington, DC, 2000 Search PubMed.
- T. Koshijima and T. Watanabe, in Association between lignin and carbohydrates in wood and other plant tissues, 2003 Search PubMed.
- G. Henriksson, M. Lawoko, M. E. E. Martin and G. Gellerstedt, Holzforschung, 2007, 61, 668–674 CrossRef CAS.
- M. Lawoko, G. Henriksson and G. Gellerstedt, Holzforschung, 2003, 57, 69–74 CrossRef CAS.
- M. Balakshin, E. Capanema and A. Berlin, in Studies in natural products chemistry, 2014, pp. 83–115 Search PubMed.
- A. U. Buranov and G. Mazza, Ind. Crops Prod., 2008, 28, 237–259 CrossRef CAS.
- K. Iiyama, T. B. T. Lam and B. A. Stone, Phytochemistry, 1990, 29, 733–737 CrossRef CAS.
- J. Ralph, R. D. Hatfield, J. H. Grabber, H. J. G. Jung, S. Quideau and R. F. Helm, in Lignin and lignan biosynthesis, 1998 Search PubMed.
- D. Y. Min, C. M. Yang, V. Chiang, H. Jameel and H. M. Chang, Fuel, 2014, 116, 56–62 CrossRef CAS.
- J. Ralph, K. Lundquist, G. Brunow, F. Lu, H. Kim, P. F. Schatz, J. M. Marita, R. D. Hatfield, S. A. Ralph and J. H. Christensen, Phytochem. Rev., 2004, 3, 29–60 CrossRef CAS.
- C. P. Neto, A. Seca, A. Nunes, M. Coimbra, F. Domingues, D. Evtuguin, A. Silvestre and J. Cavaleiro, Ind. Crops Prod., 1997, 6, 51–58 CrossRef.
- A. Björkman, in Svensk papperstidning, 1956, vol. 59, pp. 477–485 Search PubMed.
- A. Guerra, I. Filpponen, L. A. Lucia and D. S. Argyropoulos, J. Agr. Food Chem., 2006, 54, 9696–9705 CrossRef CAS PubMed.
- Z. Hu, T. F. Yeh, H. M. Chang, Y. Matsumoto and J. F. Kadla, Holzforschung, 2006, 60, 389–397 CrossRef CAS.
- J. R. Obst, Tappi J., 1982, 65, 109–112 CAS.
- H. Aimi, Y. Matsumoto and G. Meshitsuka, J. Wood Sci., 2005, 51, 252–255 CrossRef CAS.
- Y. Edashige and T. Ishii, Phytochemistry, 1998, 49, 1675–1682 CrossRef CAS PubMed.
- M. Y. Balakshin, E. A. Capanema and H. M. Chang, Holzforschung, 2007, 61, 1–7 CrossRef CAS.
- F. Peng, J. Bian, P. Peng, H. Xiao, J. L. Ren, F. Xu and R. C. Sun, J. Agr. Food Chem., 2012, 60, 4039–4047 CrossRef CAS PubMed.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and D. Crocker, Technical Report, 2008, NREL/TP-510–42618 Search PubMed.
- F. Peng, J. L. Ren, F. Xu, J. Bian, P. Peng and R. C. Sun, J. Agr. Food Chem., 2010, 58, 5743–5750 CrossRef CAS PubMed.
- J. L. Wen, S. L. Sun, B. L. Xue and R. C. Sun, Materials, 2013, 6, 359–391 CrossRef CAS.
- J. L. Wen, S. L. Sun, B. L. Xue and R. C. Sun, Holzforschung, 2013, 67, 613–627 CrossRef CAS.
- E. Hägglund, B. Lindberg and J. McPherson, Acta Chem. Scand., 1956, 10, 1160–1164 CrossRef.
- S. N. Sun, T. Q. Yuan, M. F. Li, X. F. Cao, F. Xu and Q. Y. Liu, Cell. Chem. Technol., 2012, 46, 165 CAS.
- A. U. Buranov and G. Mazza, Ind. Crops Prod., 2012, 35, 77–87 CrossRef CAS.
- M. Schwanninger, J. Rodrigues, H. Pereira and B. Hinterstoisser, Vib. Spectrosc., 2004, 36, 23–40 CrossRef CAS.
- J. L. Wen, Z. Sun, Y. C. Sun, S. N. Sun, F. Xu and R. C. Sun, J. Biobased Mater. Bioenergy, 2010, 4, 408–425 CrossRef CAS.
- R. Singh, S. Singh, K. Trimukhe, K. Pandare, K. Bastawade, D. Gokhale and A. Varma, Carbohydr. Polym., 2005, 62, 57–66 CrossRef CAS.
- J. J. Villaverde, J. Li, M. Ek, P. Ligero and A. de Vega, J. Agr. Food Chem., 2009, 57, 6262–6270 CrossRef CAS PubMed.
- J. M. Lawther, R. C. Sun and W. Banks, Ind. Crops Prod., 1996, 5, 97–105 CrossRef CAS.
- R. Sun, J. Fang, A. Goodwin, J. Lawther and A. Bolton, J. Agr. Food Chem., 1998, 46, 2817–2822 CrossRef CAS.
- S. Gupta, R. Madan and M. Bansal, Tappi J., 1987, 70, 113–116 CAS.
- M. Balakshin, E. Capanema, H. Gracz, H. M. Chang and H. Jameel, Planta, 2011, 233, 1097–1110 CrossRef CAS PubMed.
- X. Du, G. Gellerstedt and J. Li, Plant J., 2013, 74, 328–338 CrossRef CAS PubMed.
- X. Du, M. Pérez-Boada, C. Fernández, J. Rencoret, C. José, J. Jiménez-Barbero, J. Li, A. Gutiérrez and A. T. Martínez, Planta, 2014, 239, 1079–1090 CrossRef CAS PubMed.
- N. Terashima, S. A. Ralph and L. L. Landucci, Holzforschung, 1996, 50, 151–155 CrossRef CAS.
- T. T. You, L. M. Zhang, S. K. Zhou and F. Xu, Ind. Crops Prod., 2015, 71, 65–74 CrossRef CAS.
- T. Tokimatsu, T. Umezawa and M. Shimada, Holzforschung, 1996, 50, 156–160 CrossRef CAS.
- N. Takahashi and T. Koshijima, Wood Sci. Technol., 1988, 22, 231–241 CrossRef CAS.
- E. A. Capanema, M. Y. Balakshin and J. F. Kadla, J. Agr. Food Chem., 2004, 52, 1850–1860 CrossRef CAS PubMed.
- E. Capanema, B. MYu and J. Kadla, J. Agr. Food Chem., 2005, 53, 9639–9649 CrossRef CAS PubMed.
- D. Ibarra, M. I. Chávez, J. Rencoret, J. C. Del Río, A. Gutiérrez, J. Romero, S. Camarero, M. J. Martínez, J. Jiménez-Barbero and A. T. Martínez, J. Agr. Food Chem., 2007, 55, 3477–3490 CrossRef CAS PubMed.
- D. J. Yelle, J. Ralph and C. R. Frihart, Magn. Reson. Chem., 2008, 46, 508 CrossRef CAS PubMed.
- L. Zhang, G. Gellerstedt, J. Ralph and F. Lu, J. Wood Chem. Technol., 2006, 26, 65–79 CrossRef CAS.
- Z. J. Shi, L. P. Xiao, J. Deng and R. C. Sun, Bioenergy Res., 2013, 6, 1212–1222 CrossRef CAS.
- T. Q. Yuan, S. N. Sun, F. Xu and R. C. Sun, J. Agr. Food Chem., 2011, 59, 10604–10614 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |