Nanostructured molybdenum phosphide/N,P dual-doped carbon nanotube composite as electrocatalysts for hydrogen evolution†
Abstract
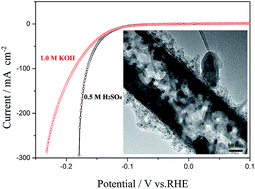
Porous or tubular electrocatalyst with a high surface area is highly desired for hydrogen evolution reaction (HER). Herein we designed and fabricated nanostructured molybdenum phosphide/N,P dual-doped carbon nanotube as electrocatalyst for HER. The electrocatalyst has a high specific surface area of 93.0 m2 g−1 and a big pore volume of 0.42 cm3 g−1, which exhibit good activity toward HER both in acidic and alkaline solutions. To drive cathodic current densities of 1 and 10 mA cm−2, the catalyst only requires overpotentials of 63 and 116 mV vs. RHE in 0.5 M H2SO4 solution, respectively, greatly smaller than those of most of catalysts reported previously. Even in 1.0 M KOH solution, to achieve the same cathodic current densities, the catalyst needs overpotentials of 62 and 117 mV vs. RHE, respectively, also smaller than those of most of catalysts reported previously. Furthermore, the catalyst exhibits excellent long-term stability for HER both in acidic and alkaline solutions. Therefore, the present strategy may open a way for the development of highly active nonprecious catalysts for the HER.

 Please wait while we load your content...
Please wait while we load your content...