Spherical CuO superstructures originating from hierarchical malachite microspheres by different post-treatment routes: a comparison study of the morphology and the catalytic property
Yuxing Zhang,
Yonghong Ni* and
Shiyong Ye
College of Chemistry and Materials Science, Key Laboratory of Functional Molecular Solids of Education Ministry, Anhui Laboratory of Molecule-Based Materials, Anhui Normal University, 1 Beijing East Road, Wuhu, 241000, P. R. China. E-mail: niyh@mail.ahnu.edu.cn; Fax: +86-553-3869303
First published on 11th January 2016
Abstract
In this paper, spherical CuO superstructures were obtained by employing freshly-produced hierarchical malachite (Cu2(OH)2CO3) microspheres as the precursor in two different post-treatment approaches. Experiments showed that spherical CuO superstructures constructed by porous nanocuboids with a mean size of ∼50 nm were prepared after the hierarchical malachite microspheres had been calcined in air at 300 °C for 2 h, while spherical CuO ones built up of abundant nanosheets with a mean thickness of ∼10 nm were formed by post-treatment of the hierarchical malachite microspheres in NaOH solution at 60 °C for 15 min. The products were characterized by XRD, SEM, HRTEM/TEM, IR and TG-DTA. It was found that CuO superstructures with different shapes presented different catalytic activities for the reduction of 4-nitrophenol to 4-aminophenol in excess NaBH4 solution and for the thermal decomposition of NH4ClO4.
Introduction
Hierarchical inorganic micro-/nano-structures constructed by smaller building units including nanoparticles, nanorods, nanobelts,1 and complex nanocrystals with a well-defined shape and inner structure2 have been attracting extensive research interest in materials science due to their novel properties and potential applications. Importantly, hierarchical structures exist widely in nature, such as the many kinds of organisms with versatile architectures.3 Hence, the synthesis and analysis of hierarchical structures will undoubtedly be helpful for a deeper understanding of nature.As one of the famous minerals, malachite is commonly used in jewellery due to its beautiful peacock green colour. Furthermore, malachite is also an important industrial raw material and has wide applications as catalysts, coatings and pigments.4 As a subcarbonate salt, however, malachite is unstable at a high temperature. Thus, it can still be employed as a copper source for the preparation of CuO with special shapes and promising applications. Many hierarchical malachite microstructures have been reported in the past decades,5–8 and CuO with hierarchical microstructures was finally obtained through the conversion of malachite. For instance, Kratohvil and Matijević investigated the formation of copper compounds with different compositions in a system with urea utilizing various Cu(II) salts as the initial copper sources.5 Amorphous spherical malachite particles were obtained when Cu(NO3)2 was used. Finally, shape-retained CuO particles were produced by calcining various copper basic compounds at 700–800 °C.5 Xu and Xue employed CuSO4 and K2CO3 as the reactants to successfully prepare hierarchical Cu2(OH)2CO3 spherical architectures through a simple hydrothermal route without the assistance of any inorganic additives or organic structure-directing reagents.6 They found that the original concentration of reactants, hydrothermal time and temperature were three important factors affecting the shape evolution of malachite crystals, and that CuO with a similar morphology could only be obtained by thermally treating the malachite in air.6 Sun and coworkers designed a controlled heating hydrated nanoparticle route to investigate the formation process of hierarchical Cu2(OH)2CO3 and CuO nanostructures, employing CuSO4 and Na2CO3 as the reactants in the presence of PVP 6000.8 Jiu et al. hydrothermally prepared hollow self-assembled Cu2(OH)2CO3 particles at 120 °C for 4 h, utilizing CuSO4 and urea as the reactants in the presences of PVP and H2O2. The as-prepared hollow particles could be converted into hollow CuO particles by calcinations at 600 °C for 3 h.7 Markedly, an appropriate temperature or additives were usually needed in the above routes. In addition, as one of the important p-type semiconductors, CuO bears good photoconductive and photochemical performances and can be used as a heterogeneous catalyst,9,10 gas sensor,11,12 optical switch,13 and magnetic storage media,14 and in lithium batteries15,16 and solar cells.17 In the abovementioned literature, however, the interest was mainly focused on the morphology of the CuO hierarchical microstructures. Correlation studies between the property and morphology were ignored.
In the present work, we design a room-temperature hydrolysis route to successfully prepare hierarchical malachite microspheres constructed by nanocuboids, which differ from previous reports,5–8 employing Cu(NO3)2·5H2O and K2CO3 as reactants without the assistance of any additives. Different from the previous reports, spherical CuO superstructures were obtained through two different approaches: pyrolysis and base treatment. It was found that the CuO superstructures prepared by the two post-treatment technologies presented different morphologies. Importantly, CuO superstructures with different morphologies exhibited different catalytic activities for the reduction of 4-nitrophenol in NaBH4 solution and the thermal decomposition of NH4ClO4 (AP).
Experimental
Materials
Ammonium perchlorate (AP) was purchased from the Jinan Henghua Chemical Co., Ltd. (>99%, AR). All of the other reagents were analytically pure, purchased from the Shanghai Chemical Company and used without further purification.Preparation of hierarchical Cu2(OH)2CO3 microspheres
To prepare the malachite precipitate, 0.2 mol L−1 Cu(NO3)2 and 0.087 mol L−1 K2CO3 solutions were prepared, respectively. In a typical procedure, 15 mL Cu(NO3)2 solution was added to 50 mL K2CO3 solution under vigorous stirring at room temperature. Here, the initial molar ratio of the Cu2+/CO32− ion was about 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. A bluish flocculent precipitate appeared at once. After ceaselessly stirring for 1 h the bluish flocculent precipitate was left, without shaking, at room temperature for 24 h. The bluish flocculent precipitate disappeared and a green precipitate was produced. The green precipitate was filtered, washed in turn with deionized water and anhydrous ethanol several times, and finally dried at room temperature.
4. A bluish flocculent precipitate appeared at once. After ceaselessly stirring for 1 h the bluish flocculent precipitate was left, without shaking, at room temperature for 24 h. The bluish flocculent precipitate disappeared and a green precipitate was produced. The green precipitate was filtered, washed in turn with deionized water and anhydrous ethanol several times, and finally dried at room temperature.
Fabrication of spherical CuO superstructures
CuO superstructures were obtained through two approaches: (1) the Cu2(OH)2CO3 precursor was calcined in air at 300 °C for 2 h; (2) the precursor was treated in 0.75 mol L−1 of 20 mL NaOH solution at 60 °C for 15 min under magnetic-stirring. Finally, the black product was collected, washed and dried under the same conditions as above.Characterization
X-ray powder diffraction (XRD) patterns were carried out on a Shimadzu XRD-6000 X-ray diffractometer equipped with Cu Kα radiation (λ = 0.154060 nm), employing a scanning rate of 0.02° s−1. Scanning electron microscopy (SEM) images were obtained on a Hitachi S-4800 field emission scanning electron microscope, employing an accelerating voltage of 5 kV. High resolution transmission electron microscopy (HR/TEM) images were recorded on a FEI Techna G20 transmission electron microscope, employing an accelerating voltage of 200 kV. The TG-DTA of the precursor was completed on a differential thermal analyzer (Thermo Electron Company of America, SDT Q600). The FTIR spectra of the products were obtained on an IR Prestige-21 Infrared spectrometer (Shimadzu Corporation). UV-vis absorption spectra were recorded on a Metash 6100 UV-vis absorption spectrophotometer (Shanghai).Catalytic study
Results and discussion
Formation and characterization of hierarchical Cu2(OH)2CO3 microspheres
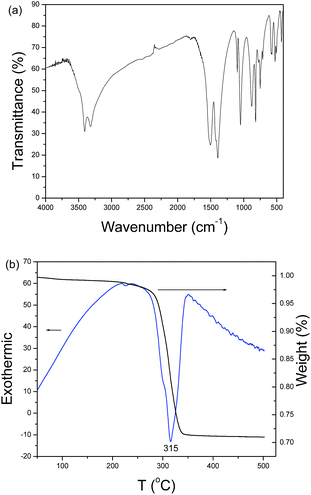
According to inorganic chemistry, CO32− ions can hydrolyze in aqueous solution to produce OH− ions. When metal ions are added to the soluble carbonate solution, three kinds of precipitates can be obtained: hydroxide, carbonate and subcarbonate. In the present system, copper subcarbonate can be prepared when Cu2+ ions and CO32− ions are mixed. Fig. 1a shows a typical XRD pattern of the product prepared after aging the mixed solution of Cu2+ ions and CO32− ions at room temperature for 24 h. All diffraction peaks can be indexed as the monoclinic malachite by comparison with the data of PDF card no. 76-0660. No impurity peaks such as Cu(OH)2 and azurite (Cu3(OH)2(CO3)2) are found, implying that the product is pure. A typical low-magnification SEM image of the product is depicted in Fig. 1b. Plentiful microspheres with a mean diameter of ∼7 μm are visible. Further enlargement shows that the microspheres are constructed by abundant nanocuboids of ∼200 nm in length (see Fig. 1c and its inset). Fig. 2a depicts the IR spectrum of the as-prepared product, which is very similar to Xu’s report.6 This further confirms the formation of malachite in the present experimental conditions. Fig. 2b exhibits the TG-DTA curves of the as-obtained product. A slight weight loss from room temperature to 250 °C can be found in the TG curve, which should be attributed to the adsorbed water on the surface of the sample. A dramatic weight loss is found in the range from 250 °C to 350 °C; accordingly, an obvious energy change in the DTA curve can also be found in the above temperature range. Based on calculation, the weight loss of the sample is ∼28%. According to the decomposition equation of malachite (see eqn (1)), the theoretical weight loss of malachite is 27.93%. The experimental value is very close to the theoretical one, which also proves the production of malachite in the current experimental conditions.| Cu2(OH)2CO3 = 2CuO + H2O + CO2 | (1) |
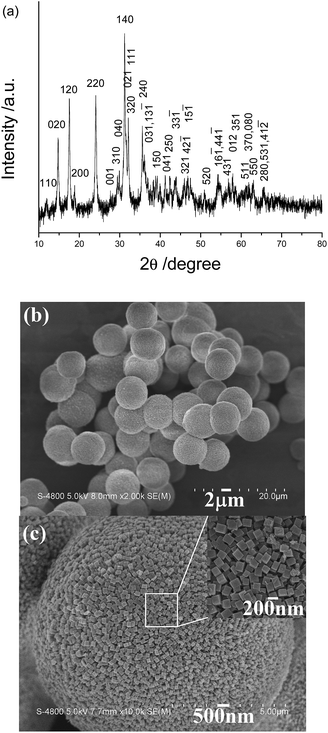 | ||
| Fig. 1 (a) XRD pattern and (b) low-magnification SEM and (c) high-magnification SEM images of the product synthesized by the present room-temperature precipitation route. | ||
Formation of spherical CuO superstructures
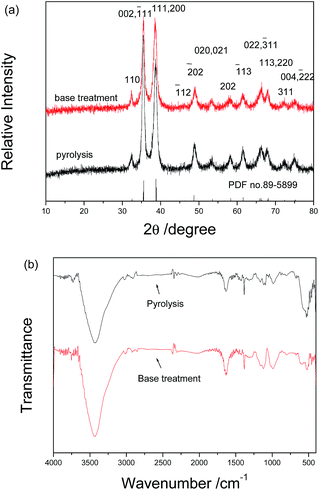
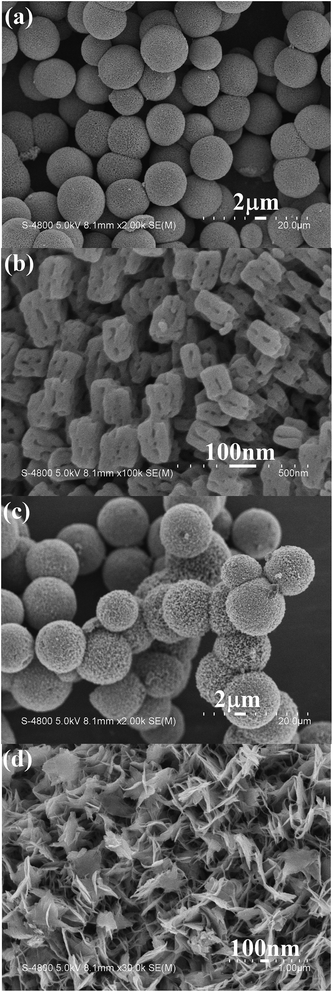
The above as-obtained hierarchical malachite microspheres can be converted into CuO through simple pyrolysis and base treatment, respectively. Fig. 3 depicts XRD patterns of the final products prepared by pyrolysis and base treatment. Obviously, the two products exhibit the same diffraction pattern but different diffraction intensities. The above fact implies that the same matter is obtained. Since high temperature can promote the growth of crystals, the pyrolysis product displays higher diffraction peak intensities than the base-treatment one. By comparison with PDF card file no. 89-5899, all diffraction peaks can be indexed as the monoclinic CuO phase. Fig. 3b shows the IR spectra of the two products. They display similar outlines and are obviously different from that of the precursor. According to Xu’s report,6 the precursor has been successfully converted into CuO under the pyrolysis or base-treatment conditions.Fig. 4 exhibits typical SEM images of the final products obtained through the simple pyrolysis and base treatment, respectively. Fig. 4a and c separately display low-magnification SEM images of the two products obtained by the two post-treatment approaches. Obviously, the final products still maintain the outlines of the precursor under the two post-treatment conditions. However, the microspheres obtained by the base treatment have bigger sizes and looser surfaces than the ones prepared by the pyrolysis. High magnification SEM images clearly show the difference on the surfaces of the two kinds of microspheres. Under the pyrolysis conditions, as shown in Fig. 4b, the final product is composed of abundant porous nanocuboids, and under the base treatment conditions the final product is constructed by nanosheets (see Fig. 4d). Undoubtedly, the different conversion processes of the precursor caused the formation of CuO with different microstructures. Under the pyrolysis conditions, the precursor decomposed according to eqn (1) to produce CuO and a great deal of gas. Since the produced gas escaped, spherical CuO superstructures constructed by porous nanocuboids were formed. In the alkali solution, however, the precursor was attacked by OH− ions. Here, CuO was formed based on the below reaction:
| Cu2(OH)2CO3 + 2OH− = 2CuO + CO32− + 2H2O | (2) |
With the progress of the above reaction, the produced CuO gradually nucleated and grew. Finally, spherical CuO superstructures built up of nanosheets were obtained.
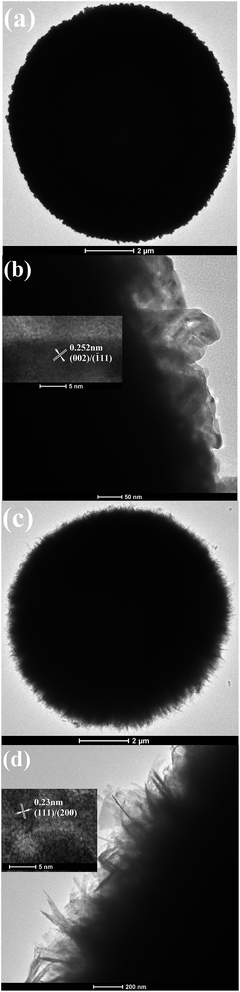
TEM images of the final products obtained by the two different post-treatment conditions are depicted in Fig. 5. As seen from Fig. 5a and c, the two products present spherical microstructures, but their surfaces are different. High magnification TEM images clearly show that under pyrolysis the surface of the microsphere consists of porous nanoparticles (see Fig. 5b); under base treatment the surface of the microsphere is made up of nanosheets (see Fig. 5d). These results are in good agreement with the results of the SEM observations. The insets given in Fig. 5b and d are HRTEM images of the surfaces of the two microspheres, respectively. The clear matrix fringes imply good crystallinities of the two products. The d-spacing of the neighboring planes is measured to be 0.252 nm for the pyrolysis product and 0.23 nm for the base-treated one, respectively. They separately match with the (002/![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11) plane and the (111/200) plane of monoclinic CuO.
11) plane and the (111/200) plane of monoclinic CuO.
Comparison of the catalytic properties
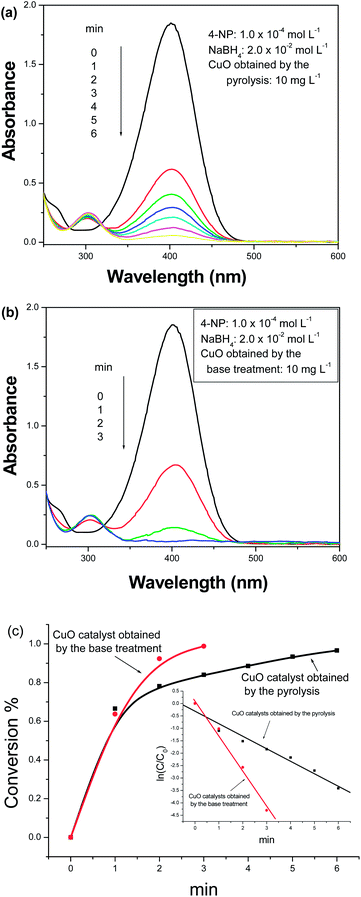
Recently, our research uncovered that CuO nanostructures possessed good catalytic activity for the reduction of some aromatic nitrocompounds such as 4-nitrophenol (4-NP), 2-nitrophenol (2-NP) and 4-nitroaniline (4-NA).18 Simultaneously, the catalytic activity was affected by the morphology and size of the CuO nanostructures. To investigate the influence of the morphology of the catalyst on the catalytic performance, in the present work, the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) in excess NaBH4 aqueous solution was selected. Fig. 6a and b depict the UV-vis absorption spectra of systems containing 4-nitrophenol and NaBH4 in the presence of spherical CuO superstructures with different morphologies for various durations. With the progress of the reaction, the peak intensities markedly decrease. However, the catalytic efficiencies of the two catalysts are different. When CuO catalysts built up of porous nanocuboids are used, only 84% of 4-NP is reduced within 3 min; when CuO catalysts constructed by nanosheets are employed, ∼99% of 4-NP is reduced within the same duration (see Fig. 6c). Markedly, CuO microspheres constructed by nanosheets exhibit stronger catalytic activity than ones built up of porous nanocuboids. The corresponding rate constants are calculated to be 8.76 × 10−3 s−1 for CuO microspheres built up of porous nanocuboids and 23.9 × 10−3 s−1 for CuO microspheres constructed by nanosheets (see inset of Fig. 6c). The catalytic ability of the CuO catalysts obtained by the base treatment is ∼3 times stronger than that of the CuO catalysts prepared by the pyrolysis. Moreover, compared with previous reports (see Table 1), the present spherical CuO superstructures also display similar or stronger catalytic activity for the reduction of 4-NP. In particular, against some noble metal catalysts such as Ag and Au, the present CuO spherical superstructures have a lower cost, which is very important in practical applications.| Catalyst and its amount | Kinetic rate constant (k) | Ratio constant (K) | Ref. |
|---|---|---|---|
| a The calculated amount of catalyst: 3 mL 10 mg L−1 CuO solution was used in the experiments. | |||
| TAC-Ag-1.0/4 mg | 5.19 × 10−3 s−1 | 1.30 s−1 g−1 | 19 |
| Ag-NP/C composite/1.0 mg | 1.69 × 10−3 s−1 | 1.69 s−1 g−1 | 20 |
| Fe3O4-@SiO2-Ag/1.0 mg | 7.67 × 10−3 s−1 | 7.67 s−1 g−1 | 21 |
| Fe3O4-@SiO2-Ag/0.02 mg | 5.50 × 10−3 s−1 | 275 s−1 g−1 | 22 |
| Fe3O4-@C@Ag/0.01 mg | 3.72 × 10−3 s−1 | 372 s−1 g−1 | 23 |
| Fe3O4-@C@Ag-Au/0.01 mg | 15.80 × 10−3 s−1 | 1580 s−1 g−1 | 23 |
| Leaf-like CuO/0.03 mg | 35.5 × 10−3 s−1 | 1180 s−1 g−1 | 18 |
| Dumbbell-like CuO/0.03 mg | 4.77 × 10−3 s−1 | 159 s−1 g−1 | 18 |
| Flowerlike CuO/0.03 mg | 10.6 × 10−3 s−1 | 353 s−1 g−1 | 18 |
| CuO (nanocuboids)/0.03 mga | 8.76 × 10−3 s−1 | 292 s−1 g−1 | This work |
| CuO (nanosheets)/0.03 mg | 23.9 × 10−3 s−1 | 797 s−1 g−1 | This work |
Furthermore, spherical CuO superstructures prepared by different post-treatment conditions also presented different catalytic abilities for the decomposition of ammonium perchlorate (AP). As shown in Fig. 7a, when no catalyst is added, the initial and final decomposition temperatures of pure AP are in turn 300 and 471 °C. After 2 wt% of CuO is added, the corresponding temperatures are in turn 262 and 358 °C for CuO prepared by pyrolysis, and 256 and 350 °C for CuO prepared by base-treatment. After introducing the CuO catalyst, the complete decomposition temperature of AP lowers by 113 and 121 °C, respectively. Accordingly, the total decomposition temperature range changes from 171 °C to 96 °C and 94 °C, respectively. Fig. 7b depicts the DTA curves of the thermal decomposition of pure AP and the AP–CuO mixture. Indeed the introduction of CuO markedly decreases the decomposition temperature of AP.
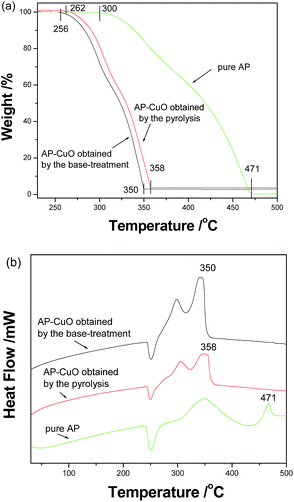 | ||
| Fig. 7 (a) The TGA and (b) DTA curves of pure AP and a mixture of AP and spherical CuO superstructures obtained by different post-treatment conditions. | ||
According to SEM observations, the spherical CuO superstructures obtained by the base treatment were composed of nanosheets, which made the final product loose. Obviously, this was favorable for contact between the catalyst and reactants. Although the escape of decomposed gases under the pyrolysis conditions led to the formation of CuO microspheres built up of porous nanocuboids, the contact area between the catalyst and reactants only slightly increased. As a result, the CuO microspheres obtained by the base treatment presented higher catalytic activity than the ones prepared by pyrolysis.
Conclusions
In summary, hierarchical malachite microspheres constructed by nanocuboids have been successfully prepared by a room-temperature aging route, employing Cu(NO3)2·5H2O and K2CO3 as initial reactants without the assistance of any additive. The as-obtained malachite microspheres could be transformed into spherical CuO superstructures through two different approaches: pyrolysis and base treatment. Experiments showed that different post-treatment routes could markedly affect the morphologies of the final CuO: spherical CuO superstructures constructed by porous nanocuboids of ∼50 nm in sizes were formed by pyrolysis at 300 °C for 2 h, and spherical CuO superstructures built up of nanosheets of ∼10 nm in thickness were obtained by the base-treatment at 60 °C for 15 min. It was found that the rate constants of the two catalysts for the reduction of 4-nitrophenol in NaBH4 solution were in turn 8.76 × 10−3 s−1 for CuO prepared by the pyrolysis, and 23.9 × 10−3 s−1 for CuO obtained by the base treatment. Also, CuO obtained by the base treatment presented stronger catalytic activity for the decomposition of NH4ClO4 than that prepared by pyrolysis. The above facts indicated that the catalytic activities of CuO superstructures could be affected by the post-treatment approaches of the precursor.Acknowledgements
The authors thank the National Natural Science Foundation of China (21171005 and 21571005) for the fund support.Notes and references
- (a) Q. Ahsanulhaq, H. J. Kim and B. Y. Hahn, Nanotechnology, 2007, 18, 485307 CrossRef; (b) Y. Shi, X. Li, J. K. Hu, J. H. Lu, Y. C. Ma and Y. H. Zhang, J. Mater. Chem., 2011, 21, 16223 RSC.
- X. Gou, G. Wang, J. Yang, J. Park and D. Wexler, J. Mater. Chem., 2008, 18, 965 RSC.
- G. Zou, H. Li, D. Zhang, K. Xiong, C. Dong and Y. Qian, J. Phys. Chem. B, 2006, 110, 1632 CrossRef CAS PubMed.
- V. Balitsky and M. Bublikova, Prog. Cryst. Growth Charact. Mater., 1990, 21, 139 CrossRef.
- S. Kratohvil and E. Matijević, J. Mater. Res., 1991, 6, 766 CrossRef CAS.
- J. S. Xu and D. F. Xue, J. Phys. Chem. B, 2005, 109, 17157 CrossRef CAS PubMed.
- H. F. Jiu, Y. X. Sun, Z. H. Li, Y. Z. Wang, Y. H. Fu, Z. J. Zhang, J. W. Zhang, X. M. Fan and L. X. Zhang, Micro Nano Lett., 2011, 6, 639 CAS.
- J. H. Sun, Y. Z. Jia, Y. Jing, Y. Yao, J. Ma, F. Gao and C. L. Xia, J. Nanosci. Nanotechnol., 2009, 9, 5903 CrossRef CAS PubMed.
- S. Gao, S. Yang, J. Shu, S. Zhang, Z. Li and K. Jiang, J. Phys. Chem. C, 2008, 112, 19324 CAS.
- J. M. Hong, J. Li and Y. H. Ni, J. Alloys Compd., 2009, 481, 610 CrossRef CAS.
- J. T. Zhang, J. F. Liu, Q. Peng, X. Wang and Y. D. Li, Chem. Mater., 2006, 18, 867 CrossRef CAS.
- G. X. Zhu, H. Xu, Y. Y. Xiao, Y. J. Liu, A. H. Yuan and X. P. Shen, ACS Appl. Mater. Interfaces, 2012, 4, 744 CAS.
- A. H. MacDonald, Nature, 2001, 414, 409 CrossRef CAS PubMed.
- R. V. Kumar, Y. Diamant and A. Gedanken, Chem. Mater., 2000, 12, 2301 CrossRef CAS.
- X. P. Gao, J. L. Bao, G. L. Pan, H. Y. Zhu, P. X. Huang, F. Wu and D. Y. Song, J. Phys. Chem. B, 2004, 108, 5547 CrossRef CAS.
- C. Wang, Q. Li, F. F. Wang, G. F. Xia, R. Q. Liu, D. Y. Li, N. Li, J. S. Spendelow and G. Wu, ACS Appl. Mater. Interfaces, 2014, 6, 1243 CAS.
- T. Maruyama, Sol. Energy Mater. Sol. Cells, 1998, 56, 85 CrossRef CAS.
- W. Che, Y. H. Ni, Y. X. Zhang and Y. Ma, J. Phys. Chem. Solids, 2015, 77, 1 CrossRef CAS.
- M. H. Rashid and T. K. Mandal, J. Phys. Chem. C, 2007, 111, 16750 CAS.
- S. Tang, S. Vongehr and X. Meng, J. Phys. Chem. C, 2010, 114, 977 CAS.
- Y. Chi, Q. Yuan, Y. Li, J. Tu, L. Zhao, N. Li and X. Li, J. Colloid Interface Sci., 2012, 383, 96 CrossRef CAS PubMed.
- K. S. Shin, Y. K. Cho, J. Y. Choi and K. Kim, Appl. Catal., A, 2012, 413–414, 170 CrossRef CAS.
- Q. An, M. Yu, Y. Zhang, W. Ma, J. Guo and C. Wang, J. Phys. Chem. C, 2012, 116, 22432 CAS.
| This journal is © The Royal Society of Chemistry 2016 |