Plasmonic photothermal cancer therapy with gold nanorods/reduced graphene oxide core/shell nanocomposites†
Abstract
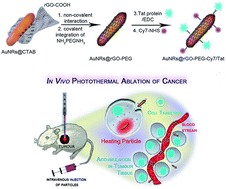
Gold nanorods (Au NRs) are known for their efficient conversion of photon energy into heat, resulting in hyperthermia and suppression of tumor growth in vitro and in vivo. Au NRs are thus of great promise for photothermal therapy (PTT) of different cancers. From the point of cancer therapy, low laser powers are essential (≤1 W cm−2) to ensure minimal side effects such as skin burning. Herein, we investigate the potential of polyethylene glycol functionalized reduced graphene oxide (rGO-PEG) enrobed Au NRs for the photothermal destruction of human glioblastoma astrocytoma (U87MG) cells in mice. We show that Au NRs@rGO-PEG are ideal multifunctional theranostic nanostructures that can exert efficient photothermal destruction of tumors in mice upon low doses of NIR light excitation and can act as fluorescent cellular markers due to the presence of a NIR dye integrated onto the rGO shell. Due to the specific interaction between Tat protein modified Au NRs@rGO-PEG nanostructures with the human glioblastoma astrocytoma (U87MG) cells, selective targeting of the tumor is achieved. In vivo experiments in mice show that upon irradiation of the tumor implanted in mice at 800 nm under low doses (0.7 W cm−2), U87MG tumor growth gets suppressed. The study demonstrates that the novel nanomaterials allow for an efficient destruction of solid tumors and might thus serve as an excellent multi-functional theranostic agent in photothermal therapeutic applications.

 Please wait while we load your content...
Please wait while we load your content...