Synthesis of luminescent squaramide monoesters: cytotoxicity and cell imaging studies in HeLa cells†
Abstract
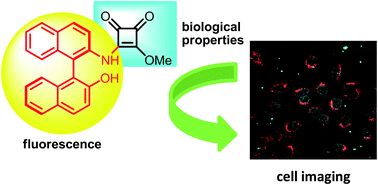
Novel luminescent squaramide monoesters functionalised with different fluorophore groups have been synthesised and explored in cell imaging for the first time. Cytotoxicity studies performed in HeLa cervical cancer cells revealed high activity for some of these novel structures, highlighting the importance of the fluorescent fragment in the efficiency of these promising anticancer agents. In addition, fluorescence cell microscopy disclosed the different biodistribution behaviour depending on the fluorescent moiety, and the possibility of nuclear localisation of chiral non planar squaramide monoesters.

 Please wait while we load your content...
Please wait while we load your content...