Theranostic liposomes containing conjugated polymer dots and doxorubicin for bio-imaging and targeted therapeutic delivery†
Abstract
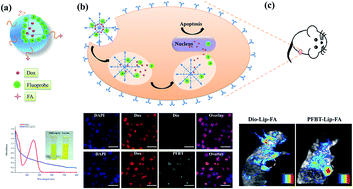
This work was devoted to the development of a lipid-based theranostic nanoparticle able to simultaneously host conjugated polymer dots, doxorubicin (Dox) and folate acid (FA). Poly(9,9-dioctylfluorene-2,7-diyl-co-benzothiadiazole) (PFBT) was chosen as the fluorescent probe because of its high brightness in in vitro cellular uptake studies and good biocompatibility in in vitro/in vivo toxicity experiments. The theranostic liposomes (PFBT–Dox–Lip–FA) exhibited a hydrodynamic size of 127.30 ± 3.20 (nm) with a zeta potential of −25.00 ± 2.00 (mV). Mostly importantly, the extent of Dox release at 24 h from PFBT–Dox–Lip–FA showed a satisfactory result under mild hyperthermia conditions compared with Dox–Lip–FA. Such rapid release led to a lower half maximal inhibitory concentration (IC50) in MCF-7 cells at 16.8 ± 4.5 (μg mL−1), whereas the IC50 of Dox–Lip–FA (37 °C) was 28.3 ± 3.7 (μg mL−1). The cellular uptake study also revealed higher drug accumulation in tumor cells for theranostic liposomes. In vivo studies of PFBT–Dox–Lip–FA on tumor-bearing mouse models revealed that the distribution of liposomes in the tumors could be indicated accurately by PFBT. Besides, tumor-bearing mice could be significantly inhibited by PFBT–Dox–Lip–FA. Together with its negligible in vivo toxicity, PFBT–Dox–Lip–FA is a useful system for simultaneous cancer diagnosis and targeted drug delivery.

 Please wait while we load your content...
Please wait while we load your content...