Dehydrochlorination of polyvinylchloride using Al-modified graphitic-C3N4†
Abstract
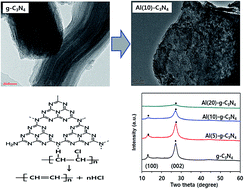
One of the efficient chemical recycling processes for waste polyvinylchloride (PVC) is the dehydrochlorination reaction using an environmentally benign solvent such as polyethylene glycol. Hydrogen chloride (HCl) is simultaneously formed through the unzipping degradation mechanism of PVC due to its lower thermal stability, this causes severe corrosion problems for the operating equipment. For the dehydrochlorination of PVC, a novel graphitic carbon nitride (g-C3N4) possessing a large amount of basic sites was prepared via melem-based thermal synthesis with an aluminum species structural modifier during the g-C3N4 preparation to verify the dehydrochlorination activity for PVC according to the aluminum content in the g-C3N4 matrix. The optimal 10 wt% aluminum modified g-C3N4 catalyst showed an excellent dehydrochlorination activity for PVC in an environmentally benign polyethylene glycol solvent due to the superior characteristics of the large amount of basic sites in g-C3N4. The positive effects of Al2O3 were mainly attributed to the incorporation of the structural modifier Al2O3 in the matrix of g-C3N4, where Al2O3 was a chemically inert material that enhanced the basicity of g-C3N4.

 Please wait while we load your content...
Please wait while we load your content...