Relay recognition of F− and a nerve-agent mimic diethyl cyano-phosphonate in mixed aqueous media: discrimination of diethyl cyanophosphonate and diethyl chlorophosphate by cyclization induced fluorescence enhancement†
Abstract
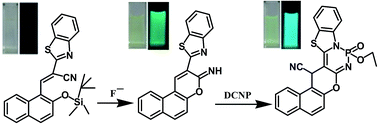
Anion to nerve agent simulant detection by relay recognition has been designed and realized for the first time with sequence specificity (F− → DCNP) via a fluorescence “off–on–on” mechanism. The discrimination of DCNP and DCP via a CIFE (cyclization induced fluorescence enhancement) mechanism has also been demonstrated here. Test strips based on the sensors with F− and DCNP are fabricated, which can act as convenient and efficient nerve agent and F− test kits. The origin of the sequence specificity of different fluorescence recognition was revealed through single X-ray crystal and NMR analysis.

 Please wait while we load your content...
Please wait while we load your content...