Design and synthesis of new ultra-low band gap thiadiazoloquinoxaline-based polymers for near-infrared organic photovoltaic application†
Abstract
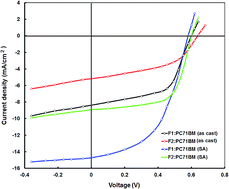
Two D–A copolymers, F1 and F2, with fluorene and thiazole units were substituted, respectively, on a thiadiazoloquinoxaline (TDQ) unit to enhance the electron-accepting strength of TDQ. The copolymers were synthesized by a cross-coupling Stille reaction and their optical and electrochemical properties were examined, which revealed that they have ultra-low band gaps and absorption in the near-infrared. These copolymers were employed as donors along with PC71BM as an electron acceptor for the fabrication of solution-processed bulk heterojunction (BHJ) polymer solar cells. After the optimization of the donor-to-acceptor weight ratio and the solvent additive (4 v% DIO as solvent additive), devices with F1:PC71BM and F2:PC71BM displayed power conversion efficiencies (PCEs) of 5.80% and 3.32%, respectively. Although F2 possesses a broader absorption profile compared with F1, the lower value of PCE for the F2-based device was attributed to the low LUMO offset between F2 and PC71BM, which limited the exciton dissociation. The abovementioned results indicate that these copolymers can be utilized for ternary BHJ and tandem solar cells to achieve a high PCE.
- This article is part of the themed collection: Editors Collection for RSC Advances - India

 Please wait while we load your content...
Please wait while we load your content...