Phase diagram for a nano-yttria-stabilized zirconia system
Abstract
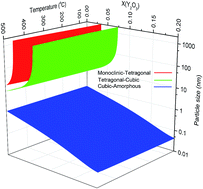
Due to the attractive properties of nanoparticles because of their effective surface area, they have been studied widely. Nano-yttria-stabilized zirconia (n-YSZ) is a ceramic which has been scrutinized extensively in past years. Because of the different stability behavior of n-YSZ in comparison with bulk YSZ, a new phase diagram is needed for the n-YSZ system in order to identify stable phases under various conditions. In this study, a phase diagram for the n-YSZ system was provided to determine phase stability ranges at room temperature with respect to particle size and composition. The calculation of phase diagrams (CALPHAD) approach was applied to calculate the Gibbs energy of bulk YSZ. It was combined with the surface energy of each phase in the n-YSZ system, i.e. monoclinic, tetragonal, cubic, and amorphous, to produce the total Gibbs energy of each individual phase of the n-YSZ system. By applying the CALPHAD approach, a 3-D phase diagram for the n-YSZ system was established in which the stability range of each individual phase can be predicted based on the particle size, composition, and temperature.

 Please wait while we load your content...
Please wait while we load your content...