Synthesis, characterization and stability of BSA-encapsulated microbubbles
Awaneesh Upadhyay and
Sameer V. Dalvi*
Chemical Engineering, Indian Institute of Technology Gandhinagar, Chandkheda 382424, India. E-mail: sameervd@iitgn.ac.in
First published on 29th January 2016
Abstract
In this work, we present an account of experimental studies performed for the synthesis, shelf stability and in vitro stability of microbubbles made from perfluorobutane (PFB) gas and coated in a shell of Bovine Serum Albumin (BSA). These microbubbles were produced by probe sonication method using formulations containing BSA, caprylic acid (CA) and N-acetyl-DL-tryptophan (Tryp) in different combinations. The freshly prepared polydisperse (0.5–20 μm) microbubble samples were then size isolated by centrifugal differentiation to produce microbubble suspensions with a narrow size range of 3 to 5 μm. Among all the different combinations of BSA, CA and Tryp used, the formulation containing BSA and Tryp yields microbubbles with a maximum shelf life (of ∼8 months). This stability was observed when microbubbles were stored in an aqueous solution (80% v/v) consisting of the original solution [which contains BSA and Tryp dissolved in phosphate buffer saline (PBS)] used to make the microbubbles, 1,2-propane-diol (10% v/v) and glycerin (10% v/v). Fluorescence and Circular Dichroism (CD) spectroscopic analyses were carried out to study the effect of additives such as CA and Tryp, and heating and sonication on the secondary and tertiary structure of BSA. It was found that the use of Tryp in the formulation favors greater unfolding of BSA as compared to CA. This in addition to a lower diffusivity of PFB, and lower solubility of BSA in the solution containing Tryp leads to a relatively stable microbubble suspension. Microbubbles produced using different BSA formulations were mixed with PBS at 37 °C to check in vitro stability of the microbubbles. It was observed that microbubbles produced from BSA and Tryp persisted longer than other microbubbles. In addition to this, in vitro ultrasonography (USG) carried out using freshly prepared microbubbles and microbubbles stored for around 4 months also yielded promising results.
Introduction
Gaseous microbubbles are gaining popularity as ultrasound contrast agents and drug delivery vehicles. A single microbubble consists of a gas core of 2–10 μm in size, encapsulated in a shell made of a layer of stabilizing molecules such as surfactants, polymers, lipids, proteins or other bio-compatible materials.1 The gaseous core, being compressible, can expand and contract when subjected to ultrasound. The rarefaction (expansion) and contraction of microbubbles upon exposure to ultrasound produce acoustic backscatter which is used for diagnostic imaging purposes.2–8 The microbubble surface can be functionalized with a targeted drug moiety which is released when microbubble fractures and/or cavitation occurs upon application of ultrasound. The other application of microbubbles is IV O2 delivery.9–12 Thus, microbubbles made of bio-compatible materials can cater to many biomedical applications and technologies.For all such applications, it is necessary to have a stable colloidal suspension of microbubbles. The stabilization is mainly provided by the shell of microbubbles which is generally made of materials such as proteins and lipids. There are several advantages/disadvantages associated with the use of a specific material as encapsulating shell. The use of polymer as a shell material can produce stable microbubbles but polymers also make a very rigid shell which may reduce the usefulness of microbubbles as ultrasound contrast agents.1,13 For last few decades, lipid and protein microbubbles have been frequently employed for contrast imaging,14,15 as drug delivery vehicles, for gene therapy16,17 and for intravenous (IV) oxygen delivery.12 Table 1 lists several microbubble formulations which are commercially available mainly for the use as contrast agents.18–22
| SN | Name | Manufacturer | Shell | Gas | Mean size | Stability | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Albunex | Molecular Biosystems Inc | 5% HSA | Air | 4 | 1 year | 18–21 |
| 2 | Optison | GE Healthcare | 1% HSA | C3F8 | 3.6–5.4 | 1 year | 18–22 |
| 3 | Definity | BMS | Phospholipid | C3F8 | 1.1–3.3 | 18 months | 21 and 22 |
| 4 | Sonovue (BR1)/Lumason | Bracco Diagnostics | Phospholipid | SF6 | 2.5 | 2 years | 21 and 22 |
| 5 | Levovist/SHU508A | Berlex Labs Inc, CA/Scherring AG, Germany | Galactose, fatty acid (palmitic acid (0.1)%) | Air | 2.7 | — | 21 |
| 6 | Imagent/AF0150 (dry microsphere) | Alliance Pharma Corp | Surfactant | Perfluorohexane/nitrogen | 6 | — | 21 |
| 7 | Sonovist/SHU563A | Scherring Germany | Cyanacrylate polymer | Air | 1 | — | 21 |
| 8 | Quantison | Andaris, Nottingham | HSA | Air | 3.2 | — | 21 |
| 9 | Sonozoid | GE healthcare | Lipid | PFB | 3 | — | 22 |
Serum albumins (human or bovine) have been used for preparation of microbubbles by many researchers. Use of albumin increases the number of surface groups inherent to albumin available for functionalization and hence such microbubbles can be easily used as multifunctional agents. Formulations like Albunex or Optison used human serum albumin (HSA) as a protein to stabilize the gas microbubbles (see Table 1). These microbubbles with mean sizes between 3–5 μm were reported to have a shelf-stability of about a year.18 “Quantison” commercialized by Andaris, Nottingham, UK (now withdrawn)20 also used HSA as a stabilizing material for gaseous microbubbles. HSA or BSA consists of amino acids as their basic building blocks. The primary structure of these proteins is the long polypeptide chain of amino acids. The amino acids in polypeptide chain arrange themselves in helical (α helix) or sheet like structures (β sheet) with several connecting turns resulting into a secondary structure of the albumin. In BSA, the majority of the secondary structure consists of α-helices (∼55–68%) with a very less β-sheet structure.23,24 This secondary structure is made of nine loops connected through 17 disulfide (S–S) bridges.23,25 Such secondary structures then coil themselves to give a tertiary structure.23 The formation of microbubble shell from albumin therefore requires unfolding of the secondary and tertiary structure of albumin molecule so that the albumin molecules interact with each other forming inter molecular linkages. The procedure for microbubble formation using albumin was described initially by the Grinstaff and Suslick.26 It was reported that heating the serum albumin to its denaturation temperature destroys intramolecular disulfide bridges resulting in free -SH groups. Sonication (after heating) used to emulsify the surrounding gas in the solution facilitates the intermolecular disulfide bond formation which then results into a microbubble shell.
Recently, several authors have demonstrated the use of BSA as a base protein to make microbubbles. Table 2 summarizes such reports.11,27–40 It can be observed that the most of BSA microbubbles suspensions are highly unstable. The maximum stability reported is around 6 months. In this work, we report preparation, characterization and stability of 3–5 μm microbubbles produced using BSA as a model protein and PFB as a core gas. These BSA microbubbles were found to be stable for around 8 months (32 weeks). CA and Tryp were used as additives in the formulation. These additives were also used in Optison formulation.41 CA and Tryp have been used to improve thermal stability of BSA42 and HSA.43–45 Variation in size distribution of BSA microbubbles with time during storage was also studied to estimate their shelf stability. Moreover, the efforts have been made to understand the effect of different storage media and the additives, CA and Tryp on the microbubbles stability. In vitro stability studies of BSA microbubbles and the in vitro ultrasonography (USG) studies were also carried out using 3–5 μm freshly prepared microbubbles and microbubbles stored in aqueous suspensions for 4 months at 4 °C.
| SN | Shell material | Core gas | Method of production | Size (μm) | Stability | Ref. |
|---|---|---|---|---|---|---|
| a PFP: perfluoropropane gas, OFP: octafluoropropane; CO2: carbon dioxide, O2: oxygen, CEHDA: co-axial electrohydordynamic atomization. | ||||||
| 1 | BSA | Air | Sonication | 4 | Not reported | 27 |
| 2 | BSA, dextrose, PAH, pDNA | PFB | Sonication | 1–5 | In vitro half life of ∼7 hours | 28 |
| 3 | BSA | O2 | Sonication | 1 to 10 (99% <7 μm) | Around 90% microbubbles were less than 3 μm & reduces to 1–2 μm in 12 days | 11 |
| 4 | BSA, PLAGA nanoparticle (also used lipid microbubbles, DSPC-PEG40S) | OFP | Sonication | 1.93 | Not reported | 29 |
| 5 | BSA, sucrose, glutaraldehyde | O2, PFP | Sonication | 2 to 5 | Half life of 30 days at 4 °C | 28 |
| 6 | BSA, saccharose | O2, PFP | Sonication | 2 to 5 | Half life of 6 months at 4 °C/14 days at 20 °C | 31 |
| 7 | BSA, dextrose | PFB | Sonication | 1–7 | More than 6 months at 5 °C | 32 |
| 8 | BSA, bovine blood plasma (propylene, glycerol) | N2 | Flow focussing microfluidic device | 10–20 | Not reported | 33 |
| 9 | BSA, DTT | Air | Sonication | 1 to 6 | Not reported | 34 |
| 10 | BSA (with glycerol, glycerol-Tween 80, and water-glutaraldehyde collection media) | Air | CEHDA | 40–800 | Half life of around 3–5 days depending on the storage media | 36 |
| 11 | BSA, dextrose (dissolved in P + G + 0.9% NaCl) | N2 | Microfluidic | >10 | Not reported | 35 |
| 12 | BSA, 1,3-propanediol | Air | CEHDA | 25–40 | Not reported | 37 |
| 13 | BSA, dextrose, polylactide nps | PFB | Sonication | 0.77 | Over 6 days | 38 |
| 14 | BSA | Air | Sonication | 0.4–0.5 | Half life of around 55 days when stored at 4 °C, 2–3 days when stored at room temp. | 40 |
Experimental
Material
Albumin from bovine serum (>98% lyophilized powder), N-acetyl-DL-tryptophan (Tryp) (>99%) and caprylic acid (CA) (minimum 99%) were purchased from Sigma-Aldrich (India). 98 wt% pure perfluorobutane (PFB) gas was obtained from Synquest Laboratories (Alachua, FL, USA) for microbubbles preparation. All these chemicals were used without any further purification. 0.01 M phosphate buffered saline (PBS) solution (Sigma-Aldrich, India) was used for preparation of BSA solutions, size isolation, and for any further processing and analysis purposes. 1,2-Propane-diol (≥99.5%) and glycerol (≥99%) were purchased from Sigma-Aldrich, India.Production, characterization and size isolation of microbubbles
A BSA solution (30 mg mL−1) of was prepared in PBS and 0.634 μL per mL of CA and 0.98 mg per mL of Tryp were added to this solution which was then preheated to a near the denaturation temperature of BSA of ∼72 °C.46,47 Perfluorobutane (PFB) gas was used as a core gas. PFB was flown over the surface of the preheated aqueous protein solution. A 20 kHz ultrasonic probe (Model 450, Sonics) was placed at the gas–liquid interface and the solution was sonicated at a power setting of 100% amplitude (105 W) for about a few seconds (∼8–10 s). After sonication, the solution was cooled immediately to 15–20 °C using an ice bath. This freshly sonicated suspension of microbubbles, was then analyzed for size and concentration using Accusizer 780AD, NICOMP Particle Sizing Systems, Santa Barbara, CA. The microbubbles solution was also subjected to the size isolation to separate 3–5 μm microbubbles from the rest of the microbubbles population as described by Feshitan et al.48 as microbubbles with size greater than 10–15 μm are not safe for blood capillary passage.14 The optical microscopy images of freshly sonicated and size isolated 3–5 μm microbubbles were obtained immediately using 100× objectives (Nikon Ni upright microscope, Nikon Corporation, Japan). Images were captured and processed using NIS-D imaging software.FE-SEM imaging of microbubbles
The size isolated 3–5 μm BSA microbubbles were kept undisturbed for about half an hour so that microbubbles float to the surface of the solution stored in a container and form a thick layer (cake). 0.1 μl of sample from this concentrated thick layer or cake was collected and air dried on a silicon chip and was then observed under FESEM (JEOL 7600F, Japan).Storage stability
Size isolated 3–5 μm microbubbles prepared using different BSA formulations (as reported in Table 3) were collected by centrifugation as a cake and stored (at 4 °C) in two different solutions. One of the solutions was the original formulation which contains 30 mg per mL BSA, 0.634 μm per mL CA and 0.98 mg per mL Tryp (from which microbubbles were prepared) and the other solution contained 10% 1,2-propane-diol (P), 10% glycerol (G), and 80% original formulation (O). The latter solution will be referred to as PGO solution in the text from here onwards. Such a combination of 1,2-propanediol and glycerol has been reported to improve microbubble stability33,35–37 and have also been used in the commercially available microbubble formulation like definity.49| Microbubbles formulations | Total concentration (microbubbles per mL) | Number-weighted diameter (μm) | Volume-weighted diameter (μm) | PI | |||
|---|---|---|---|---|---|---|---|
| Mean | Median | Mean | Median | ||||
| BSA only | As prepared | 6.95 × 109 | 1.92 | 1.29 | 34.68 | 30.42 | 7.02 |
| 3–5 μm | 4.26 × 109 | 3.60 | 3.51 | 5.94 | 4.79 | 1.20 | |
| BSA + CA | As prepared | 1.17 × 109 | 2.44 | 1.38 | 27.89 | 26.94 | 11.43 |
| 3–5 μm | 2.51 × 108 | 3.07 | 3.11 | 3.78 | 3.56 | 1.20 | |
| BSA + Tryp | As prepared | 8.8 × 109 | 1.48 | 1.06 | 10.39 | 8.56 | 7.02 |
| 3–5 μm | 2.68 × 109 | 3.44 | 3.47 | 4.15 | 3.86 | 1.20 | |
| BSA + CA + Tryp | As prepared | 6.62 × 109 | 1.73 | 1.28 | 7.59 | 6.71 | 10.39 |
| 3–5 μm | 1.53 × 109 | 3.67 | 3.61 | 4.52 | 4.02 | 1.23 | |
Estimating the extent of BSA denaturation
Aqueous solutions with different combinations of BSA, CA and Tryp such as BSA only, BSA and CA, BSA and Tryp and a mixture of BSA, CA and Tryp (Table 3) were prepared and were then divided in to two equal aliquots. One of these aliquots was used as a control sample (untreated sample) and the other was heated upto 72 °C and sonicated for ∼8–10 s (treated sample). Effect of different additives, and treatment (heating and sonication) on the denaturation of BSA was analyzed using Circular Dichroism (CD) spectroscopy, and Fluorescence Emission (FE) studies. CD analysis was performed using a Jasco J815 CD Spectrometer (Jasco, UK), between far UV range (200 to 260 nm) using a quartz cell of 2 mm path length. Fluorescence emission studies were performed using Horiba-JobinYvonFluolorog-3 spectrofluorometer with a slit-width of 1 nm at an excitation wavelength of 295 nm. Emission spectra were recorded in a wavelength range from 300 nm to 500 nm with an optical path length of 1 cm. Untreated BSA only formulation was used as control against which other fluorescence and CD measurements were compared.In vitro persistence study of microbubbles in PBS
To study in vitro persistence of 3–5 μm microbubbles in conditions similar to body fluids, the size isolated microbubbles were mixed in a heated PBS solution. In these studies, size isolated 3–5 μm microbubbles produced from four different formulations (BSA only, BSA with CA, BSA with Tryp and BSA with CA and Tryp) were used. Roughly, 5 × 107 microbubbles per mL were added to a 3 mL PBS solution kept at 37 °C. The change in concentration and size distribution of these microbubbles were estimated using Accusizer 780AD, NICOMP for a period of 30 minutes with sampling interval of 5 minutes.In vitro contrast imaging using microbubbles
10 mL PBS was collected in a dialysis tube of 25 mm flat width and 16 mm in diameter (purchased from Sigma Aldrich, India) with one end sealed. From the other end a total of freshly prepared 5 × 108/mL of size isolated microbubbles (3–5 μm in size) were injected after which the other end was sealed carefully to avoid any air trapping. Microbubbles were mixed gently by rotating the dialysis tube for about 5–10 seconds. Ultrasonographic (USG) images were then obtained using LOGIC 3 Expert, GE clinical ultrasound scanner. The B-mode images were taken with a mechanical index of 0.4 at view depth of 3 cm at 10 MHz frequency.Two different microbubbles samples were used. While one sample was the freshly prepared 3–5 μm (size isolated) microbubble population, the other was the 3–5 μm microbubble sample stored for around 4 months at 4 °C. Microbubbles used in contrast imaging studies were produced from BSA with Tryp formulation.
Results and discussion
Production, size isolation and characterization of BSA microbubbles
Table 3 presents several details about the freshly prepared and size isolated 3–5 μm microbubbles (produced using different BSA formulations) such as the number and volume weighted average sizes, concentration (microbubbles per mL) and polydispersity index (PI) (which is the ratio of mean volume-weighted size to mean number-weighted size). Fig. 1 shows typical size distributions for freshly prepared (Fig. 1A) and size isolated (Fig. 1B) microbubbles along with the corresponding optical microscopic images (Fig. 1C and D). It can be observed from Fig. 1 that the size distribution curve for freshly prepared microbubbles shows multiple peaks for various size ranges such as 1–2, 3–5, 6–8 μm as well as for sizes higher than 10 μm. However, the size isolation of this polydisperse sample results in a microbubble suspension with narrow size distribution of 3–5 μm (Fig. 1B) as evident from the lower PI of ∼1.2 for several size isolated samples (listed in Table 3). The optical microscopy images further confirm the polydispersity of freshly sonicated (Fig. 1C) and narrow size distribution of size isolated 3–5 μm microbubbles (Fig. 1D). | ||
| Fig. 1 Size distribution of (A) freshly prepared and (B) size isolated 3–5 μm BSA microbubbles, and the optical micrographs of, (C) freshly prepared and (D) size isolated 3–5 μm BSA microbubbles. | ||
Fig. 2 presents SEM images of size isolated (3–5 μm) BSA microbubbles. Air dried samples of size isolated 3–5 μm microbubbles were imaged at 5 kV at different magnifications. It was observed that even at such high voltage (5 kV) microbubble shell remains intact or do not collapse retaining their original shape.
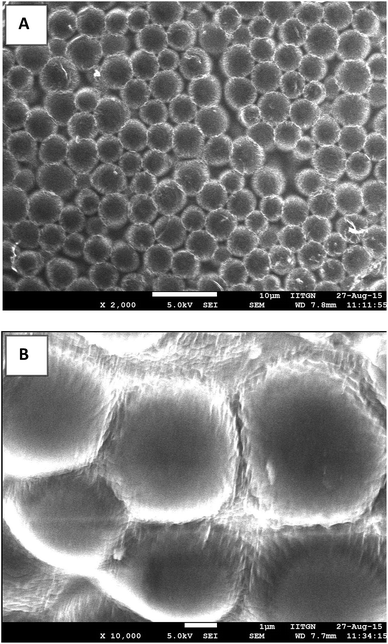 | ||
Fig. 2 FESEM images of size isolated air dried 3–5 μm BSA microbubbles prepared using BSA + Tryp formulation at (A) lower magnification (2000×) and (B) at higher magnification (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×). 000×). | ||
Storage stability of 3–5 μm BSA microbubbles
Table 4 presents details of storage stability studies carried out for several, size isolated 3–5 μm microbubble suspensions. The interesting observation that can be made (from Table 4) is the higher stability of all the microbubble suspensions stored in PGO solution as compared to the microbubbles stored in the original solutions. It can be observed from Table 4 that the 3–5 μm microbubbles produced using a solution containing BSA and Tryp and stored in PGO solution remain stable for a longer period (of ∼32 weeks or ∼8 months) as compared to microbubbles produced using any other formulation and stored in either original or PGO solution. Fig. 3 presents the variation in the size distribution of these microbubbles with time, during the storage period of 32 weeks. Fig. 3A shows the variation in number weighted size distribution whereas Fig. 3B shows variation in volume weighted size distribution of the microbubble population over a period of 32 weeks. The variations in number-weighted mean diameter and volume-weighted mean diameter over a period of 32 weeks are also shown in Fig. 3C and D respectively. It can be observed that the mean microbubble size decreases slightly during storage. The number mean size decreases from 3.5 to around 2 μm and volume mean size decreases from around 4 to 3 μm which could be attributed to the diffusion of gas from the microbubbles into the surrounding storage solution. Table 5 presents details of stability studies for these microbubbles. A slight increase in the concentration of microbubbles during the storage could be attributed to the breakage/rupture of microbubbles resulting in the detection of fractured shell material as particles during particle sizing.| Microbubble formulation | Storage media | Initial distribution | Number of days for observation | % change in concentration during observation | |
|---|---|---|---|---|---|
| Number mean | Volume mean | ||||
| a Sample dissolves completely before 30 day observation period. | |||||
| BSA | Original sol | 3.34 | 4.4 | 9a | −83 |
| PGO | 3.4 | 4.33 | 14a | −79 | |
| BSA + CA | Original sol | 2.98 | 3.68 | 7a | −70 |
| PGO | 3.07 | 3.78 | 15a | −64 | |
| BSA + Tryp | Original sol | 3.59 | 4.48 | 12a | −90 |
| PGO | 3.44 | 4.15 | 224 | −54 | |
| BSA + CA + Tryp | Original sol | 3.7 | 4.6 | 20a | −96 |
| PGO | 3.59 | 4.35 | 30 | −80 | |
| Time duration | Number weighted (μm) | Volume weighted (μm) | Relative concentration | PI (Rv/Rn) | ||
|---|---|---|---|---|---|---|
| Mean (Rn) | Median | Mean (Rv) | Median | |||
| 0 h | 3.44 | 3.47 | 4.15 | 3.86 | 1 | 1.21 |
| 1 week | 3.39 | 3.33 | 4.59 | 3.91 | 1.18 | 1.35 |
| 1 month | 3.20 | 3.15 | 4.05 | 3.76 | 1.27 | 1.27 |
| 2 months | 2.80 | 2.76 | 3.41 | 3.28 | 1.38 | 1.22 |
| 3 months | 2.98 | 2.91 | 3.84 | 3.56 | 1.25 | 1.26 |
| 4 months | 2.84 | 2.76 | 3.52 | 3.37 | 0.94 | 1.24 |
| 5 months | 2.78 | 2.68 | 3.5 | 3.33 | 0.95 | 1.26 |
| 6 months | 2.78 | 2.72 | 3.34 | 3.24 | 0.84 | 1.20 |
| 7 months | 2.58 | 2.58 | 3.14 | 3.07 | 0.53 | 1.20 |
| 8 months | 2.11 | 2.25 | 2.98 | 2.91 | 0.46 | 1.41 |
Table 4 also shows that the size isolated 3–5 μm microbubbles produced using a formulation of BSA + Tryp + CA were stable up to 30 days. However, for these microbubbles, only 20% microbubbles survived after 30 days observation period whereas for the microbubbles produced using BSA + Tryp formulation, more than 46% microbubbles survived even after 32 weeks.
These observations indicate that the additives (Tryp and CA) and the storage medium, both affect the stability of microbubble suspensions. The next two sections discuss the possible reasons behind the difference in the observed stability of different microbubble suspensions.
Effect of heating, sonication and additives on BSA denaturation and hence on the stability of microbubbles
Heating and sonication as well as the use of additives such as Tryp and CA have been reported to affect the tertiary and secondary protein structure.50,51 To study the effect of such treatment (carried out in this work) on the BSA structure, untreated, heated and treated (heated and sonicated) solutions of different combinations of BSA with CA and Tryp were analyzed using fluorescence and CD spectroscopy.A BSA molecule contains two fluorescence possessing Tryp residues, namely Tryp212 located in the hydrophobic region of BSA and Tryp134 located in the hydrophilic region of BSA.52 The fluorescence emitted by these residues can be used to understand the changes in the conformation of a protein molecule. Fig. 4 shows fluorescence emission spectra for untreated and treated (heated and sonicated) BSA formulations. It can be observed that the emission spectra for all the BSA formulations display maximum near 348 nm, indicating that these spectra correspond to the fluorescence of Tryp residues.52 The fluorescence of the treated BSA only (no additive) solution was found to decrease as compared to the fluorescence of untreated BSA only solution with a slight blue shift in the maximum. Heating of BSA molecules induces aggregation42 which reduces the fluorescence intensity.52 The decrease in fluorescence could also be attributed to the quenching of Trp134 due to a change in microenvironment.53 While for BSA only formulation, heating and sonication was found to decrease the fluorescence intensity, the fluorescence of the treated BSA with CA formulation was found to be almost the same as that of the untreated BSA only formulation. The fluorescence of treated BSA with Tryp and BSA with Tryp and CA solution was found to significantly increase as compared to the untreated as well as treated BSA only formulation. A significant increase in fluorescence upon treatment indicates that the heating and sonication could possibly have led to the higher unfolding of BSA structure and expose buried Trp212 amino acid resulting in an increased fluorescence.54 Thus, it is clear that the presence of Tryp in the BSA solution favors unfolding of BSA molecule as compared to CA indicating CA to be a better stabilizer of BSA. Anraku et al.45 also made similar observations where they found CA to be a better stabilizer of BSA against heat as compared to Tryp.
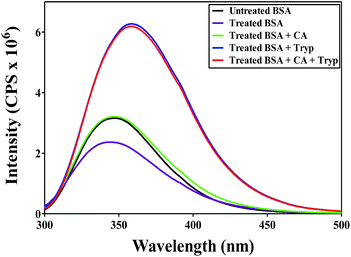 | ||
| Fig. 4 Fluorescence spectra of untreated BSA and treated (heated and sonicated) BSA formulations containing additives. | ||
Fig. 5 presents CD spectra for untreated and treated (heated and sonicated) BSA formulations. Molar ellipticity of different BSA solutions was measured between 200 and 260 nm. It is known that the two negative absorption bands at 208 and 222 nm correspond to α helices in the BSA molecule.52 It can be observed from Fig. 5 that in case of BSA only and BSA with Tryp samples the negative peak at 222 nm becomes less distinct as compared to other formulations confirming the significant structural changes in the BSA molecule when Tryp is present,51 while the presence of CA seems to inhibit any structural changes in BSA as no significant change was observed in the shape of the negative peak at 222 nm for BSA formulations containing CA. Further, the molar ellipticity at 208 nm was used to calculate % α helices using the procedure reported by Corrêa and Ramos.55 Table 6 reports the change in calculated % of α helices in BSA upon treatment (heating and sonication) in presence of Tryp and CA. The data presented in Table 6 clearly indicate that heating and sonication decreases % of α helices. While % of α helices was found to be around 70% in the untreated BSA, it decreased to 53% upon treatment. Interestingly, the use of CA was found to preserve % of α helices almost at the same level as that of untreated BSA (at 66%). On the other hand, the use of Tryp (alone or with CA) was found to decrease % of α helices to around 55–56%. This again supports the observation made through the fluorescence studies and the observation reported by Anraku et al.45 that CA is a better stabilizer of BSA as compared to Tryp and that the heating and sonication of BSA in presence of Tryp results in an increased unfolding of the secondary and tertiary structure of BSA. This can further facilitate better inter-molecular interactions among BSA molecules leading to the formation of a stable microbubble shell as compared to other formulations. A stronger and stable shell provides increased resistance to mass transfer (diffusion) of core gas (PFB) from the microbubble shell to the surrounding solution. An increase in the resistance to mass transfer decreases the rate of dissolution of microbubbles and hence the rate of change of microbubble radius as given by the equation56 below:
 | (1) |
| Sample | % α helices |
|---|---|
| Untreated BSA only | 70 |
| Heated and sonicated BSA only | 53 |
| Heated and sonicated BSA with CA | 66 |
| Heated and sonicated BSA with Tryp | 55 |
| Heated and sonicated BSA with CA and Tryp | 56 |
Effect of storage solution on the microbubble stability
The size and the number of microbubbles stored in aqueous suspensions keep changing with time due to diffusion of gas from the microbubble shell to the surrounding solution56 and the inter-bubble gas exchange.57,58 It is also possible that non-denatured BSA molecules from the microbubble shell can dissolve in the surrounding storage solution resulting in a porous microbubble shell. Such a porous microbubble shell can then result in an increased dissolution of the core gas into the surrounding solution and hence affect the microbubble storage stability. Further, the rate of microbubble dissolution is also directly proportional to the rate of diffusion of core gas (such as PFB in this work) in the storage medium.58 Therefore, the solubility of BSA and diffusivity of PFB in the storage solutions were estimated to understand their effect on microbubble stability.Table 7 reports the solubility of BSA in PBS and in PGO solutions with and without different additives. It can be observed that the solubility of BSA in PGO solutions was lower than its solubility in PBS. Also, when only Tryp is present in PGO solution, the solubility of BSA was the least (40.9 mg mL−1) as compared to other solutions containing CA or combination of CA and Tryp. The lower solubility of BSA in PGO solution containing Tryp indicates that the microbubble shell may dissolve less or slower in such solutions in comparison to other solutions, leading to a decrease in microbubble dissolution rate (as well as inter-bubble mass transfer) and hence an increased microbubble stability.58
| Media | Viscosity (cP) | Solubility of BSA (mg mL−1) | Diffusivity of PFB (×105 cm2 s−1) |
|---|---|---|---|
| PBS | 1.05 | 436 | 6.57 |
| PGO only | 1.38 | 219.7 | 4.31 |
| PGO containing CA | 2.18 | 85.9 | 2.73 |
| PGO containing Tryp | 2.32 | 40.9 | 2.56 |
| PGO containing CA and Tryp both | 2.11 | 66 | 2.82 |
Table 7 also reports the diffusivities of PFB in PBS and in PGO solutions with and without different additives. Diffusivity of PFB in pure water was obtained from literature as 6.9 × 105 cm2 s−1.59 To estimate the diffusivities of PFB in different storage (PBS and PGO) solutions, first the viscosities of these storage solutions were estimated using MCR-302 Anton Paar Rheometer at 27 °C by varying shear rate from 15 to 200 s−1 (which also are reported in Table 7). Using the inverse relationship of diffusivity with the viscosity (as given by Wilke–Chang's equation60), diffusivity of PFB in formulations containing different amount of BSA, CA and Tryp was calculated. As can be observed from Table 7, the diffusivity of PFB was highest (6.57 × 105 cm2 s−1) in PBS and the lowest in the solution containing Tryp (2.56 × 105 cm2 s−1). This is mainly because the PGO solution containing only Tryp has the highest viscosity of 2.32 cP as compared to other solutions.
The rate of dissolution of PFB from a microbubble surface can be given as follows58
| NA = kc(CAL,i − CAL,b) | (2) |
| kc = DAL/Rb | (3) |
Thus, a stable microbubble shell prepared using BSA + Tryp formulation, and the lower BSA solubility, higher solution viscosity and lower diffusivity of PFB for PGO solution containing Tryp increases the stability of microbubble suspensions due to an increase in mass transfer resistance and decrease in microbubble dissolution rates.
In vitro dissolution studies of microbubbles
Fig. 6 shows the variation in size and concentration of microbubbles of different BSA formulations when mixed with PBS maintained at 37 °C. It was observed that the microbubbles produced from different formulations have almost the same half life of ∼5 minutes. However, the persistence time of these microbubbles differ significantly. The concentration of microbubbles with BSA only, and BSA with CA formulation reduces to a near zero value within 20 minutes. The concentration of microbubbles of BSA with CA and Tryp formulation reduces to zero in 30 minutes. However, the microbubbles formed from BSA and Tryp formulation persist in the solution for a period longer than 30 minutes. For these microbubbles, a 70% decrease in concentration was obtained in 10 minutes and then the microbubbles persist at 30% concentration even after 30 minutes of observation time. This again shows that microbubbles produced from BSA and Tryp solution persist longer than the other microbubbles.Contrast imaging using BSA microbubbles
Fig. 7 shows the images captured during ultrasonography of a dialysis bag injected with and without microbubbles. It can be observed that when no microbubbles were used (only PBS solution), the image obtained was completely black with no contrast (inset to Fig. 7A) as almost no or negligible acoustic signal was being backscattered from this solution. However, contrast enhancement was observed when the BSA microbubbles were injected in the dialysis bag (Fig. 7A). Similar observations were made when 4 months old microbubble suspension was subjected for ultrasonography (Fig. 7B). These observations indicate that the BSA microbubbles produced in this work can enhance contrast during ultrasonic imaging and the effectiveness and the stability of these microbubbles does not get affected even after storage of 4 months in aqueous suspensions.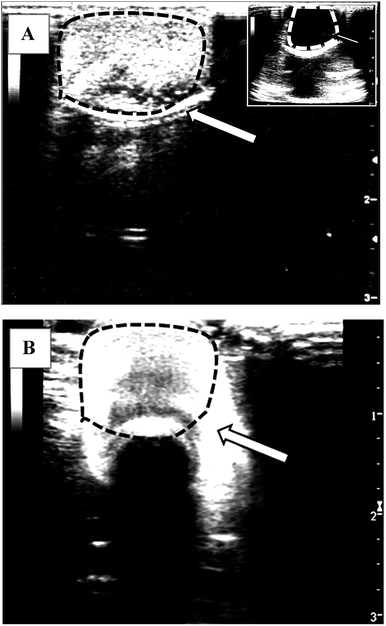 | ||
| Fig. 7 The ultrasonographic (USG) images of a dialysis tube filled with PBS containing 5 × 108/mL of size isolated (3–5 μm) microbubbles produced from BSA + Tryp formulation (A) freshly prepared microbubbles and (B) microbubbles stored for about 4 months. Inset to Fig. 7A shows USG image of dialysis tube containing only PBS solution (no microbubbles). | ||
Conclusions
In this work, we report studies on the synthesis, characterization and stability of BSA microbubble formulation with narrow size distribution (3–5 μm) and shelf stability of around 8 months. The observed stability of BSA microbubbles in this work is similar or better than the stability for BSA microbubbles reported in the literature (Table 2). The microbubbles made from the formulation containing BSA and Tryp exhibit higher storage stability (of 8 months) as compared to microbubbles made from other formulations. It is important to produce microbubble suspensions with higher stability as it not only increases the usability of the product but also minimizes the use of resources for the repeated production of microbubbles. Also, the shelf-stable microbubbles made of proteins such as BSA could be less expensive compared to the lipid based microbubbles. To understand the reasons for the increased shelf life of microbubbles made from BSA and Tryp formulation, several studies were carried out to estimate qualitatively and quantitatively, the structural changes in BSA molecules due to heating, sonication and the use of additives such as Tryp and CA. The studies included CD and fluorescence spectroscopy as well as the estimation of solubility of BSA and diffusivity of PFB in storage solution. It was found that the solubility of BSA and diffusivity of PFB in the storage solutions containing Tryp were the lowest as compared to other solutions. Also, CD and fluorescence spectroscopic studies revealed that the presence of Tryp in the solution favoured greater unfolding of BSA molecules as compared to CA. The increased unfolding of BSA molecules results in a stable microbubble shell. A stable microbubble shell prepared using BSA + Tryp formulation, the lower BSA solubility, higher solution viscosity and lower diffusivity of PFB for PGO solution containing Tryp increases the stability of microbubble suspensions due to an increase in mass transfer resistance and decrease in microbubble dissolution rates. In vitro dissolution of microbubbles in PBS solution maintained at 37 °C also which showed that microbubbles produced from BSA + Tryp formulation persist for a longer period as compared to other microbubble formulations. In vitro ultrasonography imaging studies performed reveal that these microbubbles are able to produce a good contrast during ultrasonic imaging.Acknowledgements
The authors gratefully acknowledge financial support from Indian Institute of Technology Gandhinagar (IITGN) and Department of Biotechnology (DBT), Government of India to conduct this research. Authors would like to thank Dr Vasant Rathore and Dr Manisha (Platinum Diagnostics, Ahmedabad) for their help in recording the Ultrasonography images at their clinical facilities. Authors would also like to thank Ms Komal Pandey for her help in performing experiments for estimation of solubility of BSA in different aqueous solutions.Notes and references
- S. R. Sirsi and M. A. Borden, Bubble Sci., Eng., Technol., 2009, 1, 3–17 CrossRef CAS PubMed.
- B. A. Kaufmann, J. M. Sanders, C. Davis, A. Xie, P. Aldred, I. J. Sarembock and J. R. Lindner, Circulation, 2007, 116, 276–284 CrossRef CAS PubMed.
- A. Klibanov, Journal of Nuclear Cardiology, 2007, 14, 876–884 CrossRef PubMed.
- F. S. Villanueva, E. Lu, S. Bowry, S. Kilic, E. Tom, J. Wang, J. Gretton, J. J. Pacella and W. R. Wagner, Circulation, 2007, 115, 345–352 CrossRef PubMed.
- S. Zhao, D. E. Kruse, K. W. Ferrara and P. A. Dayton, Phys. Med. Biol., 2007, 52, 2055 CrossRef CAS PubMed.
- M. A. Borden, H. Zhang, R. J. Gillies, P. A. Dayton and K. W. Ferrara, Biomaterials, 2008, 29, 597–606 CrossRef CAS PubMed.
- Y. Honda and P. J. Fitzgerald, Circulation, 2008, 117, 2024–2037 CrossRef PubMed.
- C. Z. Behm, B. A. Kaufmann, C. Carr, M. Lankford, J. M. Sanders, C. E. Rose, S. Kaul and J. R. Lindner, Circulation, 2008, 117, 2902–2911 CrossRef CAS PubMed.
- M. E. Burkard and H. D. Van Liew, J. Appl. Physiol., 1994, 77, 2874–2878 CAS.
- J. Kheir, M. A. Borden and F. X. McGowan, US Pat., US20090191244, 2009.
- E. J. Swanson and M. A. Borden, Nano LIFE, 2010, 01, 215–218 CrossRef CAS.
- E. J. Swanson, V. Mohan, J. Kheir and M. A. Borden, Langmuir, 2010, 26, 15726–15729 CrossRef CAS PubMed.
- S. H. Bloch, M. Wan, P. A. Dayton and K. W. Ferrara, Appl. Phys. Lett., 2004, 84, 631–633 CrossRef CAS.
- S. Sirsi, J. Feshitan, J. Kwan, S. Homma and M. Borden, Ultrasound Med. Biol., 2010, 36, 935–948 CrossRef PubMed.
- K. Hettiarachchi, E. Talu, M. L. Longo, P. A. Dayton and A. P. Lee, Lab Chip, 2007, 7, 463–468 RSC.
- K. Ferrara, R. Pollard and M. Borden, Annu. Rev. Biomed. Eng., 2007, 9, 415–447 CrossRef CAS PubMed.
- F. Nie, H.-X. Xu, Q. Tang and M.-D. Lu, World J. Gastroenterol., 2006, 12, 7508–7513 CAS.
- H. C. D. E. G. Jablonski, J. M. Bartlett and S. B. Podell, ed. D. O. Thompson and D. E. Chimenti, Ultrasound Contrast Agents: The Advantage of Albumin Microsphere Technology, Springer, US, 1998, vol. 17A/17B, pp. CXII, 2127 Search PubMed.
- E. Quaia, in Contrast Media in Ultrasonography: Basic Principles and Clinical Applications, ed. E. Quaia, Springer-Verlag, Berlin, Heidelberg, 2005 Search PubMed.
- R. Schlief, in Advances in Echo Imaging Using Contrast Enhancement, ed. N. C. Nanda, R. Schlief and B. B. Goldberg, Springer Science + Buisness Media Dordrecht, 1997 Search PubMed.
- S. Tinkov, Development of Ultrasound Contrast Agents for Targeted Drug and Gene Delivery, Ludwig-Maximilians-Universität, 2009 Search PubMed.
- I. C. U. Society, What is CEUS, http://www.icus-society.org/about-ceus/what-is-ceus, accessed 10 Aug 2015.
- J. R. Brown, in Albumin: Structure, Function and Uses, ed. V. M. R. O. A. Rothschild, Pergamon, 1977, pp. 27–52b Search PubMed.
- K. Murayama and M. Tomida, Biochemistry, 2004, 43, 11526–11532 CrossRef CAS PubMed.
- P. Restani, C. Ballabio, A. Cattaneo, P. Isoardi, L. Terracciano and A. Fiocchi, Allergy, 2004, 59, 21–24 CrossRef CAS PubMed.
- M. W. Grinstaff and K. S. Suslick, Proc. Natl. Acad. Sci. U. S. A., 1991, 88, 7708–7710 CrossRef CAS.
- W. Grinstaff Mark and S. Suslick Kenneth, in Macromolecular Assemblies in Polymeric Systems, American Chemical Society, 1992, vol. 493, pp. 218–226 Search PubMed.
- I. Lentacker, B. G. De Geest, R. E. Vandenbroucke, L. Peeters, J. Demeester, S. C. De Smedt and N. N. Sanders, Langmuir, 2006, 22, 7273–7278 CrossRef CAS PubMed.
- C. W. Burke and R. J. Price, J. Visualized Exp., 2010, 2145 Search PubMed.
- J. Jun, J. Shang-Yi, H. Xia and L. Wen-Ping, J. Exp. Pharmacol., 2011, 2011 Search PubMed.
- D. Yong-feng, W. Ming-xi and Z. Wen-ming, Acta Pharmaceutica Sinica, 2011 Search PubMed.
- M. J. Borrelli, W. D. O'Brien Jr, L. J. Bernock, H. R. Williams, E. Hamilton, J. Wu, M. L. Oelze and W. C. Culp, Ultrason. Sonochem., 2012, 19, 198–208 CrossRef CAS PubMed.
- A. H. Dhanaliwala, J. Chen, A. J. Dixon, A. L. Klibanov and J. A. Hossack, in World Molecular Imaging Congress, 2013 Search PubMed.
- F. Vong, Y. Son, S. Bhuiyan, M. Zhou, F. Cavalieri and M. Ashokkumar, Ultrason. Sonochem., 2014, 21, 23–28 CrossRef CAS PubMed.
- J. L. Chen, A. H. Dhanaliwala, A. J. Dixon, A. L. Klibanov and J. A. Hossack, Ultrasound Med. Biol., 2014, 40, 400–409 CrossRef PubMed.
- S. Mahalingam, M. B. J. Meinders and M. Edirisinghe, Langmuir, 2014, 30, 6694–6703 CrossRef CAS PubMed.
- W.-C. Yan, D. Tan, K. T. Pun, Y. W. Tong, V. K. Sharma and C.-H. Wang, AIChE Annual meeting, 2015, p. 2015 Search PubMed.
- M. Gauthier, Q. Yin, J. Cheng and W. D. O'Brien, J. Ultrasound Med., 2015, 34, 1363–1372 CrossRef PubMed.
- D. Wu and M. Wan, J. Controlled Release, 2015, 213, e24 CrossRef.
- T. A. M. Rovers, G. Sala, E. van der Linden and M. B. J. Meinders, Food Hydrocolloids, 2016, 52, 106–115 CrossRef CAS.
- X.-H. Wang, J. Ultrasound Med., 2011, 30, 325–332 Search PubMed.
- T. Arakawa and Y. Kita, Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol., 2000, 1479, 32–36 CrossRef CAS.
- D. Faroongsarng and J. Kongprasertkit, AAPS PharmSciTech, 2014, 15, 465–471 CrossRef CAS PubMed.
- M. W. Yu and J. S. Finlayson, J. Pharm. Sci., 1984, 73, 82–86 CrossRef CAS PubMed.
- M. Anraku, Y. Tsurusaki, H. Watanabe, T. Maruyama, U. Kragh-Hansen and M. Otagiri, Biochim. Biophys. Acta, Proteins Proteomics, 2004, 1702, 9–17 CrossRef CAS PubMed.
- İ. Gülseren, D. Güzey, B. D. Bruce and J. Weiss, Ultrason. Sonochem., 2007, 14, 173–183 CrossRef PubMed.
- E. D. Vassileva and N. S. Koseva, in Advances in Protein Chemistry and Structural Biology, ed. D. Rossen, Academic Press, 2010, vol. 80, pp. 205–252 Search PubMed.
- J. A. Feshitan, C. C. Chen, J. J. Kwan and M. A. Borden, J. Colloid Interface Sci., 2009, 329, 316–324 CrossRef CAS PubMed.
- Definity, US_PI_515987-0813, http://www.definityimaging.com, accessed 27 Aug, 2015.
- K. Takeda, A. Wada, K. Yamamoto, Y. Moriyama and K. Aoki, J. Protein Chem., 1989, 8, 653–659 CrossRef CAS PubMed.
- R. Su, W. Qi, Z. He, Y. Zhang and F. Jin, Food Hydrocolloids, 2008, 22, 995–1005 CrossRef CAS.
- S. Xia, Y. Li, Q. Zhao, J. Li, Q. Xia, X. Zhang and Q. Huang, J. Agric. Food Chem., 2015, 63, 4080–4086 CrossRef CAS PubMed.
- D. Togashi, A. Ryder and D. O'Shaughnessy, J. Fluoresc., 2010, 20, 441–452 CrossRef CAS PubMed.
- A. Pabbathi, S. Patra and A. Samanta, ChemPhysChem, 2013, 14, 2441–2449 CrossRef CAS PubMed.
- D. H. Corrêa and C. H. Ramos, Afr. J. Biochem. Res., 2009, 3, 164–173 Search PubMed.
- S. V. Dalvi and J. R. Joshi, J. Colloid Interface Sci., 2015, 437, 259–269 CrossRef CAS PubMed.
- M. Lee, E. Y. Lee, D. Lee and B. J. Park, Soft Matter, 2015, 11, 2067–2079 RSC.
- S. Sridhar, A. Patel and S. V. Dalvi, Colloids Surf., A, 2016, 489, 182–190 CrossRef CAS.
- M. A. Borden, S. Qin and K. W. Ferrara, Mol. Imaging, 2010, 425–444 Search PubMed.
- C. R. Wilke and P. Chang, AIChE J., 1955, 1, 264–270 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |



