Preponderant adsorption for chlorpyrifos over atrazine by wheat straw-derived biochar: experimental and theoretical studies†
Abstract
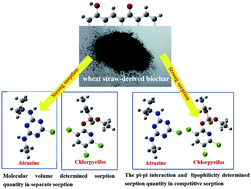
Experimental and theoretical studies have been performed for the first time to investigate the sorption of atrazine as well as the competitive sorption of the coexisting atrazine and chlorpyrifos in solution by wheat straw-derived biochar synthesized at 750 °C through oxygen-limited method (WS750). The sorption of atrazine by WS750 follows pseudo-second-order and Langmuir models. In separate sorption, WS750 has higher sorption quantity (12.0 mg g−1, 0.0556 mmol g−1) for atrazine than that (18.8 mg g−1, 0.0537 mmol g−1) for chlorpyrifos, being due to that the molecular volume (268.8 Å3) of atrazine is smaller than that (350.8 Å3) of chlorpyrifos. In competitive sorption, the sorption quantity of chlorpyrifos by WS750 is 16.5 mg g−1 (0.0470 mmol g−1), which is larger than that (7.3 mg g−1, 0.034 mmol g−1) of atrazine. This is from that chlorpyrifos has stronger pi–pi interaction with WS750 (23.68 kcal mol−1) and larger lipophilicity (log P = 4.7) than that (22.70 kcal mol−1, log P = 2.7) of atrazine. The competitive isotherm sorption of the coexisting atrazine and chlorpyrifos by WS750 can be described well by Sheindorf–Rebuhn–Sheintuch equation. This work is helpful to deep understand the sorption of bi-pollutants in water.

 Please wait while we load your content...
Please wait while we load your content...