Highly sensitive and selective ethanol and acetone gas sensors by adding some dopants (Mn, Fe, Co, Ni) onto hexagonal ZnO plates
Abstract
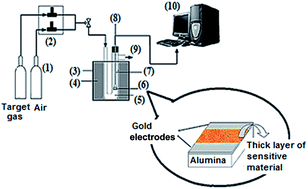
1 mol% Mn-, Fe-, Co- and Ni-doped and single phase hexagonal ZnO plates have been synthesized via a simple low temperature hydrothermal method using D-ribose as a template. The influence of the dopant species on the structural, optical and sensing properties was studied using X-ray diffraction (XRD), scanning electron microscopy (SEM), UV-vis spectroscopy, photoluminescence (PL) and a gas sensor characterization system. The results show that the dopant species have a significant effect on the morphology, crystallite size, photoluminescence and sensing properties. Co-doped ZnO shows the highest response, of 570, and selectivity to 300 ppm ethanol, compared to the other sensors. In addition, the Mn- and Ni-doped ZnO sensors show a selective response to acetone in the presence of CO and ethanol, while Fe-doped ZnO shows no considerable response to CO, ethanol and acetone gases.

 Please wait while we load your content...
Please wait while we load your content...