Biostimulation by direct voltage to enhance anaerobic digestion of waste activated sludge†
Abstract
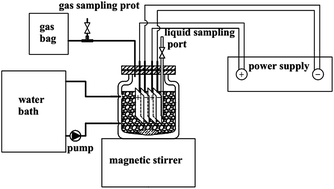
Electrical stimulation has been used conventionally for stimulation of microorganisms, and also be a promising technology to manage wastewater treatment by stimulating microbial metabolism. Previous studies on electrical stimulation were mainly focused on sewage treatment and groundwater purification, while little attention has been paid to its effect on anaerobic digestion of waste activated sludge (WAS). In this study, different voltages (0.3–1.5 V) were applied to investigate the influence of electrical stimulation on the anaerobic digestion of WAS. The results revealed that applied voltages could accelerate sludge hydrolysis and acidification process. The best performance in terms of methane production and sludge reduction was obtained with the applied voltage of 0.6 V. In this case, methane production increased by 76.2% with an enhanced VS removal rate (26.6%) compared to the control group. The energy consumption at 0.6 V could be neglected compared to the incremental energy generated from the methane. However, methane production decreased and hydrogen was produced when the applied voltage increased to 0.9 V. At higher voltages (1.2 V and 1.5 V), more soluble organic matters were released. In particular, the VFAs concentration peaked at 640 mg L−1 and 1001 mg L−1, respectively. Pyrosequencing revealed that hydrogenotrophic methanogens consisted majority of methanogen population when the applied voltage was over 0.6 V, while acetoclastic methanogens showed overwhelming dominance at 0.3 V. Moreover, 0.6 V enriched Pseudomonas for protein degradation and Methanoregula for methane generation with species richnesses of 19.1% and 53.3%, respectively.

 Please wait while we load your content...
Please wait while we load your content...