Anion-controlled morphologies and photophysical features of organic microcrystals by solid-phase anion exchange reactions
Chen Shen,
Guo-Ping Yong*,
Cong Zhang and
Ya Feng
Department of Chemistry, University of Science and Technology of China, Hefei 230026, P. R. China. E-mail: gpyong@ustc.edu.cn; Fax: +86 551-6360-1592; Tel: +86 551-6360-1584
First published on 19th January 2016
Abstract
The ability to control the morphologies and photophysical properties of organic micro/nanomaterials is of special importance. The research reported herein demonstrates a solid phase anion exchange reaction can be used as simple, low cost and green approach to tailor the morphologies of boron-difluoride complex microcrystals. The great influence of anions on the morphologies of such organic microcrystals is also discovered. The changes in morphology induce distinctive changes in the photophysical properties. Compared to one- or three-dimensional morphologies, two-dimensional hexangular microflake, rhombic and rectangle microsheet morphologies reveal higher phosphorescent quantum yields and enhanced near-infrared absorptions, which should be attributed to the morphological anisotropy of two-dimensional microsheets, demonstrating morphology-sensitive photophysical features.
Introduction
Understanding and controlling crystallization with the goal of efficiently producing particles with a specific morphology, size distribution, and structure (crystalline polymorph and degree of crystallinity) is a critical need in many industries.1–4 Controlling the shape and size of inorganic nanocrystals is important for their manufacturing and use in a range of applications.5–8 Recently, organic micro/nanomaterials have also drawn more and more attention, as a result, the fabrication and morphology control of micro- or nano-structures of organic small molecules are very important not only in fundamental science but also in the potential technological applications in many fields.9–17 Organic micro/nanostructures with various morphologies such as particles, rods, wires, sheets, cubes and fibers, have been fabricated by using different strategies. Generally, non-templated or template-induced molecular self-assemblies have been used for the fabrication of organic micro/nanostructures with variable morphologies and interesting properties.The non-templated approach mainly concerns the driving forces of assembly of the molecules themselves through hydrogen bonding, van der Waals forces, π–π stacking, or ion–π interaction, and so on. Among them, the reprecipitation method is considered as an effective and facile process to prepare organic micro/nanomaterials with unique morphology. However, morphology/size controllable fabrication of organic micro/nanomaterials is still a challenge when using this approach. Since the properties of micro-/nano-scale organic materials greatly depend on their morphological features, there is an increasing interest in developing new preparation methods to control the morphology/size of the organic micro/nanostructural materials, and further exploring the effects of the morphology on their properties. The solid-phase reaction has been known as a unique and environmental friendly strategy to fabricate organic micro/nanostructural materials.18 On the other hand, the most common method for the preparation of well-shaped micro/nanostructures is taking advantage of the confinement function of hard templates or organic surfactants. However, high cost, complicated operation and environmental malignancy of the fabrication process usually limit wide applicability of this method.
It is well known that the anions play key roles in the molecular construction and supramolecular assembly. Recently, scientists found that some anions could be utilized as capping agents, which were adsorbed selectively on a specific crystalline plane of materials to lower its surface energy, resulting in the preferential exposure of this surface in the product.19,20 Moreover, anion species show high selectivity to their preferable planes in some cases, which enabled the formation of distinct morphologies by using different anions as capping agents.21–23 However, anion-controlled morphologies of organic micro/nanomaterials are poorly reported.24 We recently reported morphology-controllable synthesis of boron-difluoride (BF2) complex microcrystals by simple solid-phase reaction methods, which exhibit interesting morphology-dependent photophysical properties.25 Herein, we unveil morphology-controllable fabrication of other BF2 complex microcrystals by facile and environmental friendly solid-phase anion exchange reactions. By using anion exchange strategy, various morphological BF2 complex microcrystals were fabricated which the morphological properties are tuned by different anions. This anion induced morphological regulation method and solid-phase anion exchange reaction are simple, low cost, green and convenient for mass production, showing great potential in the morphology-controllable fabrication of functional organic micro/nanomaterials.
Experimental
Synthesis
All of the chemicals were AR reagents obtained from commercial sources and used without further purification. The BF2 complex (BF2-HbipoCl, microcrystal A) was prepared from BF2-bipo by simple vapor–solid protonation reaction with HCl vapor, according to the previous procedure.25Characterization
Microanalytical data (C, H, N) were collected on Vario ELIII elemental analyzer. The solid-state UV/vis/NIR absorption spectra were recorded at room temperature on a DUV–3700 UV/Vis/NIR spectrometer. The solid-state photoluminescence spectra and decay lifetimes were determined at room temperature on a Fluorolog-3-TAU fluorescence spectrophotometer. The solid-state quantum yields were measured also on a Fluorolog-3-TAU fluorescence spectrophotometer equipped with a BaSO4-coated integrating sphere. Powder X-ray diffraction (PXRD) patterns were collected on a Philips X'pert PRO SUPER diffractometer operating with nickel-filtered Cu-Kα radiation (λ = 1.540598 Å) at 40 kV and 200 mA. Field-emission scanning electron microscopy (FE-SEM) measurements were performed with a FEI Sirion 200 field emission scanning electronic microanalyser operated at an accelerating voltage of 5 kV.Results and discussion
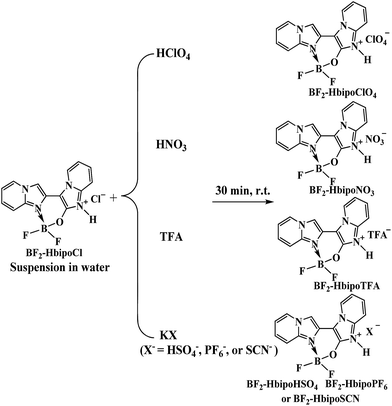
Solid-phase anion exchange reactions between BF2-HbipoCl (microcrystal A) and various polyatomic anions lead to a series of BF2-HbipoX complexes (Scheme 1). Examination of SEM micrographs of these BF2 complex microcrystals reveals the interesting and dramatic changes in morphology upon variation in the polyatomic anions associated with the BF2-Hbipo cation (Fig. 1 and 2). The initial microcrystal A (BF2-HbipoCl) discloses two-dimensional (2D) hexangular microflake morphology25 (Fig. 1a). However, when Cl− anion in microcrystal A was exchanged with two kinds of polyatomic anions (PF6− and SCN−) by the solid-phase anion exchange reactions (immersing BF2-HbipoCl to the saturated KPF6 and KSCN aqueous solution), the resultant microcrystals B (BF2-HbipoPF6) and C (BF2-HbipoSCN) present 2D rhombic microsheet (Fig. 1b) and 2D rectangle microsheet (Fig. 1c) morphologies, respectively. From SEM images, one can see that the sheetlike microcrystals A–C possess thickness of about 100–200 nm. Obviously, different anions play important roles in controlling the morphologies of organic microcrystals. | ||
| Fig. 1 SEM images of (a) hexangular microflakes of microcrystal A, (b) rhombic microsheets of microcrystal B, and (c) rectangle microsheets of microcrystal C. | ||
 | ||
| Fig. 2 SEM images of (a) microstrips of microcrystal D, (b) microrods of microcrystal E, (c) microbricks of microcrystal F, and (d) microblocks of microcrystal G. | ||
The anion-induced morphological changes are further demonstrated by some oxygen-containing polyatomic anions (HSO4−, ClO4−, NO3− and TFA−). Using these polyatomic anions to exchange Cl− anion in microcrystal A via solid-phase anion exchange reactions, these oxygen-containing polyatomic anions also induce interesting and dramatic changes in morphology of microcrystals D (BF2-HbipoHSO4), E (BF2-HbipoClO4), F (BF2-HbipoNO3) and G (BF2-HbipoTFA). The microcrystals D and E are found to form one-dimensional (1D) microstrip (Fig. 2a) and 1D microrod (Fig. 2b) morphology, respectively. In contrast, the microcrystals F and G reveal three-dimensional (3D) brick-like (Fig. 2c) and 3D block-like (Fig. 2d) microstructure, respectively. It is interestingly noted that oxygen-containing polyatomic anions with four oxygen atoms induce 1D microstructures, whereas, oxygen-containing polyatomic anions with less than four oxygen atoms result in 3D morphologies. The results indicate that the anion exchange induced morphological changes are relative to the types of polyatomic anions, and even the numbers of oxygen atoms in the oxygen-containing polyatomic anions, implying the anions really play key roles in controlling the morphologies.
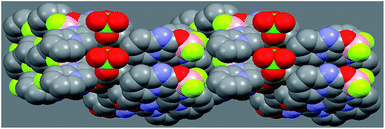
The sharp peaks in the powder X-ray diffraction (PXRD) patterns confirm microstructures of microcrystals A–G to be highly crystalline (Fig. 3). The distinct change in morphology of these organic microstructure materials is attributed to ordered repetition of the molecular stacking and/or supramolecular interaction. In microcrystal E, the molecules form 1D double-stranded chain through supramolecular interactions, which is further assembled into a 2D layer with relatively plane arrangement.25 The interlayer hydrogen bond (N4⋯O2′ of 2.939 Å) and short atomic contact (C5⋯O2′ of 3.414 Å) link 2D layer to form quasi-1D columnar stacking structure,25 as shown in Fig. 4. Thus, the molecular arrangements in microcrystal E should have the tendency to aggregate into a 1D columnar structure in nature,26 which is probably relative to the generation of 1D rodlike morphology of microcrystal E. The interlayer supramolecular interactions, that the oxygen atoms in ClO4− anion participate in, result in 1D rodlike morphology of microcrystal E (Fig. 2b), therefore, the supramolecular interactions by using oxygen atoms in HSO4−, NO3− and TFA− could induce 1D microstrips of microcrystal D (Fig. 2a), 3D microbricks of microcrystal F (Fig. 2c) and 3D microblocks of microcrystal G (Fig. 2d). Whereas, the absence of such oxygen atoms in microcrystals A–C makes them tend to only form 2D molecular arrangement, which favors the generation of 2D sheetlike morphology of microcrystals A–C (Fig. 1).
 | ||
| Fig. 3 PXRD patterns of (a) 2D sheetlike microcrystals A, B and C; (b) 1D or 3D microcrystals D, E, F and G. | ||
Generally speaking, organic microcrystals can reveal morphology-dependent properties.25 Although all microcrystals A–G emit blue light with strong emission peak around 455 nm, as indicated by their photoluminescence (PL) spectra (Fig. 5), the changes in morphology induce interesting changes in their phosphorescent quantum yields (PHQYs). Take microcrystals A, B and F for examples, the phosphorescent nature of microcrystals A–G is confirmed by microsecond-order decay lifetime in solid state (Fig. 6). The PHQYs of 2D hexangular microflakes of microcrystal A, 2D rhombic microsheets of microcrystal B, and 2D rectangle microsheets of microcrystal C are 42.6 ± 4%, 36.2 ± 3% and 40.5 ± 4% respectively, upon excitation at 365 nm. Whereas, 1D microstrips of microcrystal D, 1D microrods of microcrystal E, 3D microbricks of microcrystal F, and 3D microblocks of microcrystal G possess lower phosphorescent quantum yields with PHQYs of 33.1 ± 3%, 31.5 ± 3%, 20.8 ± 2%, and 11.2 ± 1% respectively, upon excitation at 365 nm. Various PHQYs of microcrystals A–G should be ascribed to their different morphology effects. Compared to 1D or 3D microcrystals D, E, F and G, 2D hexangular microflakes of microcrystal A, 2D rhombic microsheets of microcrystal B, and 2D rectangle microsheets of microcrystal C exhibit higher PHQYs, which should be attributed to the local field enhancement effect induced by the anisotropic microsheet structure.28 Generally, 2D micro/nanosheets possess higher quantum yield than that of 1D micro/nano structural organic materials.26,29
 | ||
| Fig. 5 Normalized PL spectra of microcrystals A (a), B (b), C (c), D (d), E (e), F (f), and G (g), upon excitation at 365 nm. | ||
 | ||
| Fig. 6 The decay lifetime curves of microcrystals A (a), B (c) and F (b) at emission peak of about 455 nm in solid state. The lifetime (τ) is defined as the time in which the emission intensity decays to 1/e of the initial intensity (Io), where e is the natural log constant and is equal to 2.718 (I = Ioe−(t/τ) ≥ τ = t ≥ I = (1/e)Io).27 | ||
Microcrystals A–G display another unusually morphology-sensitive photophysical phenomenon: in solid state, their absorbance even occurs in the near-infrared (NIR) region with multiple absorption bands from 1125 nm to 2500 nm (Fig. 7), which can be ascribed to the aggregation-induced NIR absorption.30,31 Interestingly, compared to 1D or 3D microcrystals D, E, F and G (Fig. 7b), 2D hexangular microflakes of microcrystal A, 2D rhombic microsheets of microcrystal B, and 2D rectangle microsheets of microcrystal C reveal not only a new absorption band around 1950 nm (Fig. 7a, asterisks), but the obviously enhanced absorption in the NIR region as well (Fig. 7a). These NIR absorption features are also attributed to morphological anisotropy of 2D microsheets, demonstrating morphology-sensitive NIR absorption features.
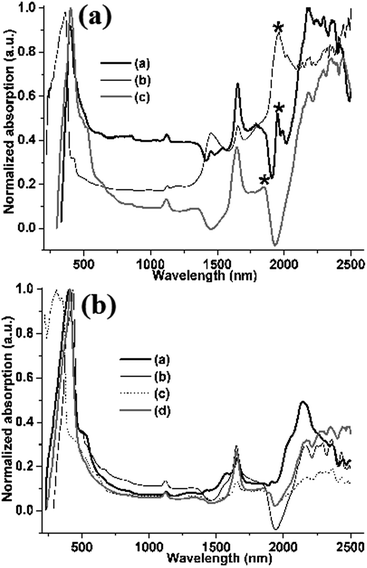 | ||
| Fig. 7 UV/vis/NIR absorption spectra: (a) 2D sheetlike microcrystals A (a), B (b) and C (c); (b) 1D or 3D microcrystals D (a), E (b), F (c) and G (d). | ||
Conclusions
In summary, a series of boron-difluoride complex microcrystals have been fabricated through a simple and green solid-phase anion exchange reaction, in which the exchanging anions show crucial influence on the morphologies of the organic microcrystals. The oxygen-containing polyatomic anions induce 1D microstrip, 1D microrod, 3D microbrick, and 3D microblock morphologies. Whereas, other polyatomic anions without oxygen atoms result in the formation of 2D rhombic and rectangle microsheet morphologies. These results indicate the species of anions play important roles in the morphological regulation of organic microstructures. The changes in morphology induce distinctive changes in the photophysical properties. Compared to 1D or 3D morphology, 2D rhombic and rectangle microsheet morphologies reveal higher phosphorescent quantum yield and enhanced NIR absorption, which should be attributed to morphological anisotropy of 2D microsheets, demonstrating morphology-sensitive photophysical features. The importance of the presented results evidences the photophysical features are sensitive to the morphology of organic micro/nanomaterials. This work is considered a significant contribution towards controlling the morphology by the anions and simple solid-phase reactions. We believe that the solid-phase anion exchange reaction opens a new direction in the field of morphology-controllable fabrication of organic micro/nanomaterials which may be potential candidates for applications as organic light-emitting diode, photovoltaics, optoelectronic devices, and so on.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (grant 21172210).Notes and references
- C. Burda, X. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025–1102 CrossRef CAS PubMed.
- N. Tian, Z. Y. Zhou, S. G. Sun, Y. Ding and Z. L. Wang, Science, 2007, 316, 732–735 CrossRef CAS PubMed.
- A. Kubacka, M. Fernandez-Garcia and G. Colon, Chem. Rev., 2012, 112, 1555–1614 CrossRef CAS PubMed.
- Y. B. Guo, L. Xu, H. B. Liu, Y. J. Li, C. M. Che and Y. L. Li, Adv. Mater., 2015, 27, 985–1013 CrossRef CAS PubMed.
- A. Selloni, Nat. Mater., 2008, 7, 613–615 CrossRef CAS PubMed.
- G. Leem, S. Sarangi, S. Zhang, I. Rusakova, A. Brazdeikis, D. Litvinov and T. R. Lee, Cryst. Growth Des., 2009, 9, 32–34 CAS.
- J. Chang and E. R. Waclawik, RSC Adv., 2014, 4, 23505–23527 RSC.
- J. Zhao, P. Yang, H. S. Chen, J. Li, Q. D. Che, Y. N. Zhu and R. X. Shi, J. Mater. Chem. C, 2015, 3, 2539–2547 RSC.
- F. J. M. Hoeben, P. Jonkheijm, E. W. Meijer and A. P. H. J. Schenning, Chem. Rev., 2005, 105, 1491–1546 CrossRef CAS PubMed.
- D. H. Kim, D. Y. Lee, H. S. Lee, W. H. Li, Y. H. Kim, J. I. Han and K. Cho, Adv. Mater., 2007, 19, 678–682 CrossRef CAS.
- Y. B. Lim, K. S. Moon and M. Lee, J. Mater. Chem., 2008, 18, 2909–2918 RSC.
- X. J. Zhang, C. Dong, J. A. Zapien, S. Ismathullakhan, Z. H. Kang, J. S. Jie, X. H. Zhang, J. C. Cheng, C. S. Lee and S. T. Lee, Angew. Chem., Int. Ed., 2009, 48, 9121–9123 CrossRef CAS PubMed.
- H. L. Dong, S. D. Jiang, L. Jiang, Y. L. Liu, H. X. Li, W. P. Hu, E. J. Wang, S. K. Yan, Z. M. Wei, W. Xu and X. Gong, J. Am. Chem. Soc., 2009, 131, 17315–17320 CrossRef CAS PubMed.
- C. S. Huang, Y. L. Li, J. E. Yang, N. Cheng, H. B. Liu and Y. J. Li, Chem. Commun., 2010, 46, 3161–3163 RSC.
- H. J. Kim, S. K. Kang, Y. K. Lee, C. Seok, J. K. Lee, W. C. Zin and M. Lee, Angew. Chem., Int. Ed., 2010, 49, 8471–8475 CrossRef CAS PubMed.
- S. Varughese, J. Mater. Chem. C, 2014, 2, 3499–3516 RSC.
- W. R. Cho, X. M. Liu, J. Forrest, J. D. Fowler and E. M. Furst, ACS Appl. Mater. Interfaces, 2015, 7, 15707–15715 CAS.
- H. B. Liu, Y. L. Li, S. Q. Xiao, H. Y. Gan, T. G. Jiu, H. M. Li, L. Jiang, D. B. Zhu, D. P. Yu, B. Xiang and Y. F. Chen, J. Am. Chem. Soc., 2003, 125, 10794–10795 CrossRef CAS PubMed.
- H. G. Yang, C. H. Sun, S. Z. Qiao, J. Zou, G. Liu, S. C. Smith, H. M. Cheng and G. Q. Lu, Nature, 2008, 453, 638–641 CrossRef CAS PubMed.
- C. Z. Wen, J. Z. Zhou, H. B. Jiang, Q. H. Hu, S. Z. Qiao and H. G. Yang, Chem. Commun., 2011, 47, 4400–4402 RSC.
- Q. Wu, F. Zhang, P. Xiao, H. S. Tao, X. Z. Wang, Z. Hu and Y. N. Lue, J. Phys. Chem. C, 2008, 112, 17076–17080 CAS.
- Q. Wu, J. X. Chen, F. Zhang, P. Xiao, Y. N. Lu, X. Z. Wang and Z. Hu, CrystEngComm, 2012, 14, 3397–3403 RSC.
- J. E. Millstone, W. Wei, M. R. Jones, H. J. Yoo and C. A. Mirkin, Nano Lett., 2008, 8, 2526–2529 CrossRef CAS PubMed.
- A. N. Jordan, S. Das, N. Siraj, S. L. de Rooy, M. Li, B. El-Zahab, L. Chandler, G. A. Baker and I. M. Warner, Nanoscale, 2012, 4, 5031–5038 RSC.
- G. P. Yong, C. Shen, C. Zhang and Y. M. Zhao, J. Mater. Chem. C, 2015, 3, 9048–9052 RSC.
- Y. F. Zhao, X. Y. Mu, C. X. Bao, Y. Fan, J. Y. Zhang and Y. Wang, Langmuir, 2009, 25, 3264–3270 CrossRef CAS PubMed.
- K. C. Stylianou, R. Heck, S. Y. Chong, J. Bacsa, J. T. A. Jones, Y. Z. Khimyak, D. Bradshaw and M. J. Rosseinsky, J. Am. Chem. Soc., 2010, 132, 4119–4130 CrossRef CAS PubMed.
- D. K. Zhang, X. Y. Hu, R. N. Ji, S. C. Zhang, J. H. Gao, Z. Y. Yan, E. Z. Liu, J. Fan and X. Hou, J. Non-Cryst. Solids, 2012, 358, 2788–2792 CrossRef CAS.
- B. K. An, S. H. Gihm, J. W. Chung, C. R. Park, S. K. Kwon and S. Y. Park, J. Am. Chem. Soc., 2009, 131, 3950–3957 CrossRef CAS PubMed.
- A. D'Aléo, D. Gachet, V. Heresanu, M. Giorgi and F. Fages, Chem.–Eur. J., 2012, 18, 12764–12772 CrossRef PubMed.
- G. P. Yong, Y. M. Zhao, Y. Feng and X. R. Zhang, J. Mater. Chem. C, 2013, 1, 3395–3398 RSC.
| This journal is © The Royal Society of Chemistry 2016 |