Electrogenerated chemiluminescence of the tris(2,2′-bipyridine)ruthenium(II)/aliphatic amine system: a universal effect of perchlorate salts†
Steffen Czioska and
Zuofeng Chen*
Shanghai Key Lab of Chemical Assessment and Sustainability, Department of Chemistry, Tongji University, Shanghai 200092, China. E-mail: zfchen@tongji.edu.cn
First published on 11th January 2016
Abstract
Sensitivity is a key parameter for electrogenerated chemiluminescence (ECL) analysis. We describe here a simple way to enhance the ECL of Ru(bpy)32+ (bpy = 2,2′-bipyridine) by adding concentrated perchlorate salts. Observations are made for a variety of aliphatic amines as coreactants in a wide pH range from pH 5 to 12 with ECL enhanced by 1.5–6.6 times for different ECL routes. By contrast, the addition of other salts such as nitrate, sulfate and chloride decreases ECL to some extent. Experimental studies suggest that perchlorate anions could stabilize the coreactant cation radicals by formation of ion pairs, which allows ECL to occur in a thicker reaction layer by a longer travel distance of these intermediates. However, other anions may get involved in detrimental redox processes. For example, nitrate and sulfate may directly react with the highly reducing coreactant free radicals or Ru(bpy)3+ intermediates, and chloride may be concomitantly oxidized at the electrode surface during ECL emission generating highly reactive HClO/ClO− intermediates. This study is important for both mechanistic fundamentals and the realistic applications of ECL.
Introduction
Electrogenerated chemiluminescence (ECL) is one of the most commonly used methods in clinical analysis such as immunoassay and DNA analysis.1–5 One of the oldest and best investigated systems is Ru(bpy)32+ (bpy = 2,2′-bipyridine) with tri-n-propylamine (TPrA) or other aliphatic amines as coreactants.6–11 While the sensitivity of the ECL technique remains a restrictive factor for its large-scale commercial success, several emission routes have been proposed on the basis of the coreactant oxidation.6–11 In the catalytic pathway with concentrated Ru(bpy)32+ and dilute TPrA, Ru(bpy)32+ is first oxidized to Ru(bpy)33+ which subsequently oxidizes TPrA to its cation radical (TPrA˙+).| Ru(bpy)32+ − e− → Ru(bpy)33+ (E0 ∼ 1.02 V vs. SCE) | (1) |
| Ru(bpy)33+ + TPrA → Ru(bpy)32+ + TPrA˙+ | (2) |
In the presence of dilute Ru(bpy)32+ and concentrated TPrA, TPrA oxidation alters to mainly proceed following the direct oxidation route:
| TPrA − e− → TPrA˙+ (E0 ∼ 0.8 V vs. SCE) | (3) |
Under the solution conditions, TPrA cation radical will undergo a rapid deprotonation, which leads to the formation of a highly reducing intermediate, TPrA free radical (TPrA˙). The excited state is produced by the energetic electron transfer between Ru(bpy)33+ and TPrA˙:
| TPrA˙+ → TPrA˙ + H+ | (4) |
| Ru(bpy)33+ + TPrA˙ → Ru(bpy)32+* + P1 | (5) |
With facile TPrA oxidation, there is an additional ECL pathway referred to as the low-oxidation-potential (LOP) ECL, which requires no oxidation of Ru(bpy)32+ with ECL generated at a potential below 1.0 V vs. SCE.12 The generation of LOP ECL requires the intermediacy of TPrA˙+ and TPrA˙ with appropriate redox potentials related to Ru(bpy)32+ couples.
| Ru(bpy)32+ + TPrA˙ → Ru(bpy)3+ + P1 | (6) |
| Ru(bpy)3+ + TPrA˙+ → Ru(bpy)32+* + TPrA | (7) |
Based on the mechanism established, considerable efforts have been made to improve the sensitivity of the Ru(bpy)32+/aliphatic amine system. For example, the modification of the electrode surface with nonionic fluorosurfactants could render the electrode surface more hydrophobic,13 which facilitates TPrA oxidation leading to ECL enhancement by an unprecedented ∼50 times via the LOP ECL pathway.14,15 Further enhancing the ECL intensity beyond this level presents a formidable challenge in analytical performance, which in turn requires a more in-depth understanding on reaction details that affects the ECL emission.
Here, we report that simply adding perchlorate salts leads to enhanced Ru(bpy)32+ ECL emission of different pathways by 1.5–6.6 times with a variety of aliphatic amines as coreactants in a wide pH range from pH 5 to 12. The discovery of such a universal enhancement effect is striking and unique which is not observed for other salts such as nitrate, sulfate and chloride salts. A possible mechanism on the enhancement effect is proposed by perchlorate stabilization of coreactant cation radicals. By contrast, other anions are either reactive with the highly reducing TPrA˙ or Ru(bpy)3+ intermediates, or oxidizable during the positive potential scan of ECL, both of which adversely lead to ECL decrease.
Results and discussion
Perchlorate salt effect on ECL of Ru(bpy)32+/TPrA system
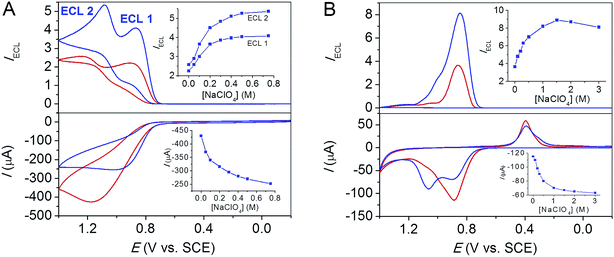
As demonstrated in previous reports, the predominant ECL route of the Ru(bpy)32+/TPrA system could be altered by changing the solution and electrode materials,16–21 and the NaClO4 effects on different ECL routes could be examined separately. In the presence of a trace amount of Ru(bpy)32+ and concentrated TPrA, the direct oxidation of TPrA at the electrode plays a predominant role in generating ECL intermediate radicals, i.e. TPrA˙+ and TPrA˙. At a freshly polished glassy carbon electrode, the cyclic voltammogram (CV) displayed a broad oxidation wave of TPrA, Fig. 1A. During the positive potential scan, two ECL waves appeared at 0.9 V and 1.23 V, resulting from the formation of excited state Ru(bpy)32+* via eqn (7) (LOP ECL) and (5) (direct coreactant oxidation ECL), respectively. With addition of 0.75 M NaClO4, TPrA oxidation is decreased due to a decreased diffusion coefficient of TPrA in the salt-concentrated solution that is more viscous. By contrast, ECL intensity for both waves is enhanced by ∼2 times. The dependence of TPrA oxidation and ECL intensity on NaClO4 concentration is shown in Fig. 1A inset. At [NaClO4] > 0.5 M, there is clear evidence for saturation kinetics for ECL intensity with NaClO4 concentration, which presumably indicates an ion-pair mechanism as discussed below.A similar effect of NaClO4 on the LOP ECL signal was also observed at an FSO-modified gold (FSO-Au) electrode. As demonstrated in earlier studies, the adsorption of the nonionic fluorosurfactant molecules at the gold electrode would facilitate TPrA oxidation by rendering the electrode surface more hydrophobic and inhibiting the growth of the surface oxides in the potential region below 1.0 V.14,15 As a result, a sharp LOP ECL peak appeared in the potential range between 0.8 and 1.0 V with a relatively low TPrA concentration (10 mM). As observed at a glassy carbon electrode, Fig. 1B shows that TPrA oxidation current is slightly decreased due to the decreased diffusion coefficient of TPrA with added concentrated NaClO4, while the LOP ECL intensity was increased by ∼2.5 times. Such an enhancement is impressive which is achieved on the basis of an original 50-time enhancement by modification of fluorosurfactants14,15 and thus represents a 125-time enhancement than that at a bare gold electrode. The LOP ECL intensity as a function of NaClO4 concentration is shown in Fig. 1B inset. These results indicate that the enhancement effect of NaClO4 is independent on both electrode materials and ECL routes.
NaClO4 effect was also examined in a solution containing relatively concentrated Ru(bpy)32+ (1 mM) and dilute TPrA (1 mM). Under this condition, the ECL reaction proceeded mainly via the catalytic pathway, i.e., TPrA was catalytically oxidized by electrogenerated Ru(bpy)33+, eqn (1) and (2). Unfortunately, it was found that concentrated Ru(bpy)32+ is not soluble in solution of concentrated perchlorate salts with Ru(bpy)3(ClO4)2 precipitated out from solution. In addition, for ECL immunoassays and DNA assays with Ru(bpy)32+ as a probe, the concentration of Ru(bpy)32+ is typically much lower than that of the amine coreactant.22,23 Therefore, our investigation of the NaClO4 effect is limited to the direct coreactant oxidation route and LOP ECL route by keeping the concentration of Ru(bpy)32+ in micromole.
The effect of NaClO4 was also observed at other pH where ECL emission is not favorable. The deprotonation of TPrA˙+ is pH-dependent which generates the key intermediate reductant TPrA˙. In earlier reports, it has been demonstrated that both direct coreactant oxidation ECL and LOP ECL exhibited strong dependence on solution pH with the maximum emission at around neutral pH.8,15 Slightly decreasing pH to 5 or increasing pH to 10 results in a rapid drop of ECL intensity. Improving the sensitivity of ECL in a wide pH domain would be beneficial to the application of ECL in a complex solution matrix. Fig. 2 shows that the LOP ECL intensity at an FSO-Au electrode increases with added NaClO4 at both pH 5 and pH 10 by a factor of 2.5 and 3, respectively. Similar phenomena were observed for both direct coreactant oxidation ECL and LOP ECL at a glass carbon electrode with addition of NaClO4 at both pH 5 and pH 10.
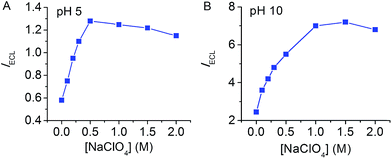 | ||
| Fig. 2 Dependence of the LOP ECL intensity on NaClO4 concentration. Electrode, FSO-Au electrode; solution, 0.15 M PBS at pH 5 (A) and pH 10 (B) with 1 μM Ru(bpy)32+ and 10 mM TPrA. | ||
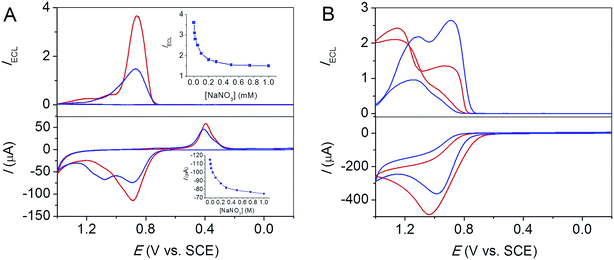
The salts effect on ECL enhancement is specific to perchlorate. Addition of other salts such as nitrate, sulphate and chloride shows negligible enhancement or even decrease in ECL intensity. Fig. 3A shows that addition of 1 M NaNO3 leads to LOP ECL decrease at an FSO-Au electrode by a factor of 2.5 at pH 7.5. Similar phenomenon was observed with added Na2SO4 at a glass carbon electrode at pH 7.5, Fig. S1.† These observations in turn also indicate that the ECL enhancement is unlikely caused by Na+ ions, which exist in all examined salts, NaClO4, NaNO3 and Na2SO4. Examination of salts with counter cations other than Na+ fails because of solubility issues, e.g., both KClO4 or Li3PO4 (PO43− is one of the components of phosphate buffer electrolyte) are poorly soluble (0.11 M for KClO4 and 3 mM for Li3PO4 at 25 °C) in water, which make both K+ and Li+ salts unsuitable for the ECL test.
Interestingly, Fig. 3B shows that addition of NaCl leads to an increase in LOP ECL at a low potential and a decrease in the direct coreactant oxidation ECL at a high potential. The different effect of NaClO4, NaNO3 and Na2SO4 on ECL, as well as the opposite effect of NaCl on different ECL routes are related with the nature of these anions as discussed in the next section.
The change of the ECL intensity is not due to the influence of added salts on the lifetime or quantum efficiency of the excited state of Ru(bpy)32+. A series of photoluminescence measurements were conducted with Ru(bpy)32+ in phosphate buffer solution of different pH, or with further addition of TPrA. No substantial changes in the photoluminescence intensity were observed before and after addition of the examined salts NaClO4, NaNO3, NaSO4 and NaCl, Fig. S2.† Earlier studies have shown that the photoredox quenching of Ru(bpy)32+ by quenchers such as MV2+, HgII or TlIII is dependent on the ionic strength and the nature of added anions by the so-called primary kinetic salt effect.24,25 However, the anions alone, such as ClO4−, NO3−, SO42− and Cl− do not quench Ru(bpy)32+ photoluminescence at neutral pH. The quenching by these anions is possible only at highly acidic pH, e.g., HClO4 as an oxidative acid is known to quench Ru(bpy)32+ yielding Ru(bpy)33+ with kq = 1.0 × 106 M−1 s−1.24,26 In any case, our experiments and the literature reports both exclude the possibility for the ECL enhancement arising from the enhancing emission of the excited state of Ru(bpy)32+.
Mechanism discussions
Since the added salts exert negligible influence on the emission of the excited state of Ru(bpy)32+, the change in coreactant ECL intensity is considered to relate with the nature and fate of coreactant intermediate radicals. Earlier studies indicated that the coreactant ECL might be affected by reactions between the quencher molecules and the ECL intermediate radicals.18,19 Besides the excited-state quenching, these side reactions would consume the intermediate radicals leading to the reduction of the ECL intensity. On the contrary, increasing the quantity of these intermediate radicals or their lifetime may enhance the ECL intensity.Based on the experimental observations and discussions above, we speculate that formation of an ion pair between TPrA˙+ cation radical and ClO4− anion may occur by coulombic attraction, eqn (8). This would stabilize TPrA˙+ cation radical by polarization resulting in a higher lifetime of this species. It has been reported that the half-life of TPrA˙+ is around 0.2 μs in neutral aqueous solution, and its travel distance is only around 6 μm.12 This indicates that the ECL emission occurs only within a very thin layer near the electrode surface. The prolonged lifetime of TPrA˙+ cation radical would allow a longer travel distance and thus increase both the spatial and temporal existence of itself and its deprotonated product, TPrA˙ free radical. Subsequently, this would increase the chance of reactions of TPrA˙+ and/or TPrA˙ intermediates with Ru(bpy)33+/2+/+ species, leading to an enhanced ECL emission. Notably, the increase in the spatial and temporal existence of TPrA˙+ and/or TPrA˙ intermediates will benefit ECL emission of different routes. As mentioned above, there is clear evidence for saturation kinetics with increasing ClO4− concentration, consistent with the ion-pair mechanism with electrogenerated TPrA˙+ all paired at high ClO4− concentrations. Beyond the point, further increase in ClO4− concentration results in negligible enhancement in ECL emission.
| TPrA˙+ + ClO4− → {TPrA˙+,ClO4−} | (8) |
The unique enhancement effect of perchlorate related to other salts is attributable to its high redox stability during ECL process. For its wide electrochemical potential window, perchlorate salts are commonly used as an electrolyte in both oxidation and reduction electrochemical experiments. Fig. S3A† shows that addition of 1 M NaClO4 in phosphate buffer at pH 7.5 results in negligible change in CV profile during both anodic and cathodic potential scans until the solvent background decomposition. By contrast, salts like NO3− and SO42− may get involved in detrimental redox reactions during ECL process. Fig. S3B and S3C† show that both NO3− and SO42− are electrochemically reduced beginning at −1.2 V vs. SCE. This observation indicates that these anions can be chemically reduced by the highly reducing TPrA˙ free radical (E0 ∼ −1.7 V vs. SCE)27,28 or Ru(bpy)3+ (E0 ∼ −1.52 V vs. SCE).29 The decrease in the quantity of TPrA˙ free radical would lead to suppressed ECL emission.
Fig. S3D† shows that Cl− is oxidizable at the electrode surface from 1.12 V vs. SCE. Interestingly, this onset potential for Cl− oxidation is located at a point between LOP ECL and the direct coreactant oxidation ECL, which accounts for the opposite effect of NaCl on the two ECL pathways in Fig. 3B. Since LOP ECL occurs at the potential prior to Cl− oxidation, the formation of an ion pair between Cl− (like ClO4−) and TPrA˙+ can stabilize TPrA˙+ leading to enhanced LOP ECL emission. By contrast, the direct coreactant oxidation ECL occurs overlapping with Cl− oxidation which generates HClO/ClO− intermediate. The presence of highly active HClO/ClO− intermediate during ECL emission is deleterious by its reaction with both TPrA˙+ and TPrA˙ resulting in ECL decrease.
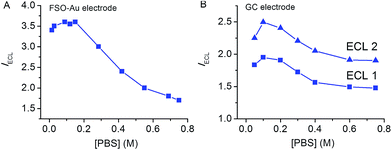
It is worth to note that the coulombic interaction should also exist between phosphate buffer anion HPO42− and TPrA˙+ cation radical. However, as shown in Fig. 4A, the LOP ECL intensity decreases with increasing phosphate buffer concentration at [PBS] > 0.1 M. This is probably because HPO42− anion also functions as a base which deprotonates TPrA˙+ instead of stabilizing it, note eqn (9). A similar effect of phosphate buffer concentration was also observed at a freshly polished glass carbon electrode, Fig. 4B. In this case, both LOP ECL and direct coreactant oxidation ECL decrease with increasing phosphate buffer concentration at [PBS] > 0.1 M.
| TPrA˙+ + HPO42− → {TPrA˙+,HPO42−} → TPrA˙ + H2PO4− | (9) |
Extension to other aliphatic amines
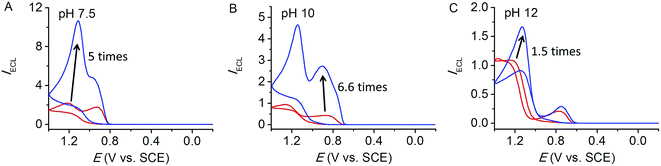
Ru(bpy)32+/TPrA ECL assays have been widely used for over decades. Despite its exclusive popularity, TPrA is toxic and volatile, and usually used at high concentrations (usually up to 100 mM) to attain good sensitivity; it is basic requiring high concentrations of acidic phosphate solutions to prepare concentrated neutral solutions of TPrA. There are continuous efforts to find effective coreactant alternatives to TPrA.30,31 Herein, we also investigated the influence of added NaClO4 on ECL of other potential amine coreactants. We anticipate that, by sensitivity improvement with NaClO4, some amines that are less efficient and unsuitable for ECL in the past may become viable for real application.With the shorter chain length of the alkyl arms, tri-n-ethylamine (TEtA) is much more soluble than TPrA. With TEtA as coreactant, the effect of perchlorate can be investigated with pH extended to 12 even with 100 mM TEtA. As shown in Fig. 5, addition of perchlorate improves the ECL by 5 and 6.6 times at pH 7.5 and 10, respectively, while the enhancement is only 1.5 times at pH 12. The observation of small enhancement at pH 12 is presumably due to competition from OH− anion which deprotonates TEtA˙+ and compromises the stabilization effect of ClO4− anion, eqn (10). This result is consistent with the negative effect of phosphate buffer concentration discussed in Fig. 4. The other structurally related coreactant tri-n-butylamine (TBuA) was also investigated. At pH 7.5, addition of NaClO4 to a solution of 10 mM TBuA (note that 100 mM TBuA is not soluble at pH 7.5) leads to an ECL enhancement by 2 times, Fig. S4.†
| TEtA˙+ + OH− → {TEtA˙+,OH−} → TEtA˙ + H2O | (10) |
Earlier work has shown that tertiary amines with hydroxy substituent can also act as efficient coreactants for Ru(bpy)32+ ECL, which are usually much less toxic and less volatile but more soluble than TPrA.30,31 The hydroxy group can promote the oxidation of amines especially at a hydrophilic electrode and substantially increase the ECL intensity. However, Ru(bpy)32+ ECL with these alkylol amines usually do not exhibit LOP ECL signal probably because the electron-withdrawing hydroxy group lowers the reductive potential of the amine free radical,32 which thus is not capable to reduce Ru(bpy)32+ to Ru(bpy)3+, note eqn (6). It is essential to bring the ECL potential down if these alkylol amines are poised to be more competitive coreactants.
TEOA is a typical alkylol amine that is structurally related to TEtA. As shown in Fig. 6, addition of 1 M NaClO4 results in ECL enhancement by 5 times with TEOA as coreactant. In addition, it can be clearly seen that the potential for the ECL maxima shift negatively by 200 mV from 1.3 V to 1.1 V upon addition of NaClO4. The nature of the ECL is unchanged and is not due to a newly appeared LOP ECL emission which is supposed to take place, if any, along with TEOA oxidation beginning from 0.8 V. Instead, the potential shift is probably due to perchlorate anion stabilization of the high-oxidation-state Ru(bpy)33+ by forming an ion pair, {Ru(bpy)33+,nClO4−}, eqn (11), which facilitates the oxidation of Ru2+ to Ru3+. Although no direct evidence is available for the ion pair formation under the experiment condition with 1 M NaClO4 and 1 μM Ru(bpy)32+, the assumption is tenable considering the concentration difference by a factor of 10−6 between Ru(bpy)32+ and ClO4− and the strong combination between them as indicated by the low solubility of [Ru(bpy)3](ClO4)2. The observation of potential shift for the direct coreactant oxidation ECL of Ru(bpy)32+/TEOA with addition of NaClO4 is indubitable since there is no LOP ECL pre-wave to interfere in this case. It also prompts us to scrutinize the available data of other amine coreactants in terms of the potential shift in ECL emission upon addition of NaClO4. From Fig. 1A with TPrA, Fig. 5 with TEtA, and Fig. S4† with TBuA, it is clear that the peak potentials of LOP ECL are nearly unchanged upon addition of NaClO4, while those for direct coreactant oxidation ECL all shift negatively by 50–200 mV, the latter consistent with the mechanism by forming {Ru(bpy)33+,nClO4−} ion pair in eqn (11).
| Ru(bpy)33+ + nClO4− → {Ru(bpy)33+,nClO4−} | (11) |
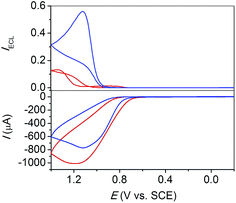 | ||
| Fig. 6 CV and ECL curves of 1 μM Ru(bpy)32+ and 100 mM TEOA at a glass carbon electrode without (red) and with (blue) 1 M NaClO4. Solution, 0.15 M PBS at pH 7.5; scan rate, 100 mV s−1. | ||
The generality of the perchlorate effect on Ru(bpy)32+ ECL was further investigated by using other alkylol amines and secondary amines as coreactants. The observations are summarized in Table 1 with CV and ECL curves shown in Fig. S5–S8.† Enhanced ECL emissions were observed with all these amines by a factor of 1.5–3.6, accompanied with a negative potential shift of around 150–200 mV for the direct coreactant oxidation pathway. These results further presage a general effect of perchlorate salts on ECL.
| TPrA | TEtA | TBuA | TEOA | DBAE | BEOA | DPrA | TMeA | |
|---|---|---|---|---|---|---|---|---|
| a Enhancement factors for the LOP ECL and the direct coreactant oxidation ECL are separated with the “/”. The “--” represents no corresponding ECL signals. TPrA: tri-n-propylamine; TEtA: tri-n-ethylamine; TBuA: tri-n-butylamine; TEOA: tri-n-ethylanolamine; DBAE: 2-(dibutylamino)ethanol; BEOA: butyl(ethanol)amine; DPrA: dipropylamine; TMeA: tri-n-methylamine. | ||||||||
| pH 7.5 | 1.8/2.1 | 2.8/5 | 4.5/2.8 | --/4.2 | 2.4/2.1 | --/1.7 | --/3.6 | --/1.5 |
Conclusions
We demonstrate that simply adding NaClO4 leads to Ru(bpy)33+ ECL enhancement by 1.5–6.6 times with a variety of aliphatic amines as coreactants in a wide pH range from pH 5 to 12. Experimental studies suggest that this universal enhancement effect arises from ClO4− stabilization of the coreactant cation radical by forming an ion pair, which extends the lifetime and travel distance of the coreactant cation radical, and allows ECL occurring in a thicker reaction layer. The ClO4− anion may also stabilize the high-oxidation-state Ru(bpy)33+ which accounts for the negative potential shift during the direct coreactant oxidation ECL emission. By contrast, addition of other salts such as NaNO3, Na2SO4 and NaCl results in ECL decrease to some extent due to the detrimental side reactions. For example, nitrate and sulfate may directly react with the ECL intermediates such as the highly reducing coreactant free radicals or Ru(bpy)3+, while chloride may be synchronously oxidized at the electrode surface during ECL emission which generate highly reactive HClO/ClO− intermediates and thus suppress ECL emission. The effect of perchlorate is universal in improving the sensitivity of the Ru(bpy)32+/aliphatic amine ECL technique and the sensitivity improvement may also lead to development of coreactants alternative to TPrA which are less toxic and more environmental friendly.Conflict of interest
The authors declare no competing financial interest.Acknowledgements
Z.-F. C. thanks the National Natural Science Foundation of China (21405114, 21573160), The Recruitment Program of Global Youth Experts by China, and Science & Technology Commission of Shanghai Municipality (14DZ2261100) for support.References
- L. Hu and G. Xu, Chem. Soc. Rev., 2010, 39, 3275 RSC.
- K. Fähnrich, M. Pravda and G. Guilbault, Talanta, 2001, 54, 531 CrossRef.
- L. Ge, J. Yan, X. Song, M. Yan, S. Ge and J. Yu, Biomaterials, 2012, 33, 1024 CrossRef CAS PubMed.
- A. Knight, Trends Anal. Chem., 1999, 18, 47 CrossRef CAS.
- X. Wang, P. Dong, W. Yun, Y. Xu, P. He and Y. Fang, Biosens. Bioelectron., 2009, 24, 3288 CrossRef CAS PubMed.
- X. Zhou, D. Zhu, Y. Liao, W. Liu, H. Liu, Z. Ma and D. Xing, Nat. Protoc., 2014, 9, 1146 CrossRef CAS PubMed.
- G. Blackburn, H. Shah, J. Kenten, J. Leland, R. Kamin, J. Link, J. Peterman, M. Powell, A. Shah, D. Talley, S. Tyagi, E. Wilkins, T. Wu and R. Massey, Clin. Chem., 1991, 37, 1534 CAS.
- J. Leland and M. Powell, J. Electrochem. Soc., 1990, 137, 3127 CrossRef CAS.
- M. Richter, Chem. Rev., 2004, 104, 3003 CrossRef CAS PubMed.
- W. J. Miao, Chem. Rev., 2008, 108, 2506 CrossRef CAS PubMed.
- R. Forster, P. Bertoncello and T. Keyes, Annu. Rev. Anal. Chem., 2009, 2, 359 CrossRef CAS PubMed.
- W. Miao, J. Choi and A. J. Bard, J. Am. Chem. Soc., 2002, 124, 14478 CrossRef CAS PubMed.
- C. Cha and Y. Zu, Langmuir, 1998, 14, 6280 CrossRef CAS.
- F. Li and Y. Zu, Anal. Chem., 2004, 76, 1768 CrossRef CAS PubMed.
- Y. Zu and F. Li, Anal. Chim. Acta, 2005, 550, 47 CrossRef CAS.
- K. Honda, M. Yoshimura, T. N. Rao and A. Fujishima, J. Phys. Chem. B, 2003, 107, 1653 CrossRef CAS.
- D. Wang, L. Guo, R. Huang, B. Qiu, Z. Lin and G. Chen, Sci. Rep., 2014, 5, 7954 CrossRef PubMed.
- H. Zheng and Y. Zu, J. Phys. Chem. B, 2005, 109, 12094 Search PubMed.
- H. Zheng and Y. Zu, J. Phys. Chem. B, 2005, 109, 16047 CrossRef CAS PubMed.
- Y. Zu and A. J. Bard, Anal. Chem., 2000, 72, 3223 CrossRef CAS PubMed.
- B. Factor, B. Muegge, S. Workman, E. Bolton, J. Bos and M. M. Richter, Anal. Chem., 2000, 73, 4621 CrossRef.
- W. Miao and A. J. Bard, Anal. Chem., 2004, 75, 5825 CrossRef PubMed.
- W. Miao and A. J. Bard, Anal. Chem., 2004, 76, 5379 CrossRef CAS PubMed.
- K. Kalyanasundaram and M. Neumann-Spallart, Chem. Phys. Lett., 1982, 88, 7 CrossRef CAS.
- G. Laurence and V. Balzani, Inorg. Chem., 1974, 13, 2976 CrossRef CAS.
- M. I. C. Ferreira and A. Harriman, J. Chem. Soc., Faraday Trans. 2, 1979, 75, 874 RSC.
- R. Y. Lai and A. J. Bard, J. Phys. Chem. A, 2003, 107, 3335 CrossRef CAS.
- E. M. Gross, P. Pastore and R. M. Wightman, J. Phys. Chem. B, 2001, 105, 8732 CrossRef CAS.
- D. M. Roundhill, in Photochemistry and Photophysics of Metal Complexes, ed. J. J. P. Fackler, Plenum Press, New York, 1994, p. 165 Search PubMed.
- X. Liu, L. Shi, W. Niu, H. Li and G. Xu, Angew. Chem., Int. Ed., 2007, 46, 421 CrossRef CAS PubMed.
- X. Yin, B. Sha, X. Zhang, X. He and H. Xie, Electroanalysis, 2008, 20, 1085 CrossRef CAS.
- J. Grodkowski and P. Neta, J. Phys. Chem. A, 2000, 104, 4475 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra24111a |
| This journal is © The Royal Society of Chemistry 2016 |