Photovoltaic properties of 3,3′-(ethane-1,2-diylidene)-bis(indolin-2-one) based conjugated polymers†
Abstract
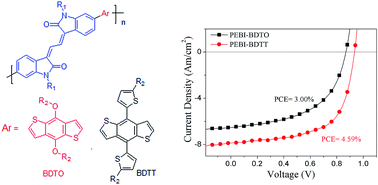
3,3′-(Ethane-1,2-diylidene)-bis(indolin-2-one) (EBI) is a π-conjugated structure in which two oxindoles are connected by a 1,3-butadiene. An N-alkylated EBI monomer has been synthesized in this study. NMR results showed that the two central double bonds of the EBI monomer were in a Z–Z configuration. Two new polymers with EBI and benzodithiophene (BDT) as building blocks have been synthesized. The new polymers possess relatively low HOMO energy levels, suitable LUMO energy levels and broad absorption through most of the visible region and extended into the near-IR. The polymers have been applied as donor materials in bulk hetero-junction polymer solar cell (PSC) devices. The PSC devices based on the blend of EBI-BDT polymers and PC71BM achieved a power conversion efficiency of 4.59% and a Voc of 0.94 V.

 Please wait while we load your content...
Please wait while we load your content...